Abstract
Purpose
Maternal cortisol levels in pregnancy may support the growth of or adversely affect fetal organs, including the brain. While moderate cortisol levels are essential for fetal development, excessive or prolonged elevations may have negative health consequences for both the mother and the offspring. Little is known about predictors of altered hypothalamic–pituitary–adrenal (HPA) axis activity during pregnancy. This study examined maternal hair cortisol concentration (HCC) in the 3rd trimester of pregnancy in relation to severe psychopathology.
Methods
Hair samples were collected from 69 women, 32 with a lifetime diagnosis of severe mental disorders (bipolar I or II disorder, moderate or severe depressive disorder, schizophrenic spectrum disorder), and 37 non-clinical controls. Hair samples were collected during the 3rd trimester, and liquid chromatography tandem mass spectrometry was used for cortisol assessment. Psychiatric diagnosis and current level of symptomatic functioning were assessed using the structured clinical interview from the DSM-5 and the global assessment of functioning scale.
Results
Women with a lifetime diagnosis of severe mental illness had significantly elevated HCC compared to controls. Poorer current symptomatic functioning was also significantly associated with elevated HCC in pregnancy.
Conclusions
The implications of alterations in HCC on both maternal and infant health need further study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A large body of research has established an intergenerational transmission of risk of psychopathology in offspring of parents with severe mental illness (SMI), such as bipolar disorder, psychosis, and depression (Bifulco et al. 2002; Willcutt and McQueen 2010; Uher et al. 2014; Liu et al. 2015). Meta-analytic findings indicate that the heritable risk of developing SMI is best perceived as a general increased risk for any mental disorder more than a diagnosis-specific risk (Rasic et al. 2013). Recently, dysregulation of the human stress regulatory system has been suggested as a potential common factor across various SMIs that may be involved in the intergenerational transmission of psychopathology risk from mother to offspring.
The prenatal period is a time of elevated vulnerability to stress for the mother-to-be (reviewed in Khoury et al. 2023). Furthermore, growing evidence suggests that maternal stress during pregnancy can have long-term effects on fetal development (Zijlmans et al. 2015). Prenatal stress may lead to long-term changes in offspring regulation of the hypothalamic–pituitary–adrenal axis (HPA) as well as other negative developmental and health outcomes of the offspring (De Weerth and Buitelaar 2005; Van den Bergh et al. 2020; Andreasen et al. 2023). As such, the effect of prenatal stress could constitute an important risk factor for developing psychopathology later in life.
Several studies have assessed the change in hair cortisol concentration (HCC) levels during pregnancy and found a consistent pattern of circulating cortisol increasing through pregnancy (reviewed in Mustonen et al. (2018)). Maternal cortisol levels triple in the 3rd trimester (Jung et al. 2011), and the fetus is more exposed to the cortisol as the enzyme activity of 11beta HSD 2 is reduced in the 3rd trimester (Seckl and Holmes 2007). This makes pregnancy an important time for exploring the status of the HPA axis in women with psychopathology (Wikenius et al. 2016; Broeks et al. 2021).
HPA axis activity may be assessed by serum, saliva, or urinary cortisol, and recently, hair cortisol has emerged as a novel approach for measuring long-term cortisol exposure. A single hair sample provides an integrated measure of chronic cortisol production, where 1 cm of hair provides a measure of one month’s cortisol production (Kirschbaum et al. 2009). Hair cortisol levels in pregnancy can be a useful marker of overall maternal HPA activity during each trimester (Anna-Hernandez et al. 2011). In previous studies, primiparity was associated with higher cortisol levels compared to multiparous women (Andersen et al. 2019).
Psychopathology has been variously associated with both HPA axis hypo- and hyperactivity from samples outside pregnancy (Staufenbiel et al. 2013). Anxiety and PTSD are associated with decreased cortisol levels, major depressive disorder, bipolar disorder, psychosis, and schizophrenia have been linked with increased HCCs (Staufenbiel et al. 2013; Borges et al. 2013). However, we still need more knowledge of HPA axis functioning in people with severe psychopathology especially during the sensitive time of pregnancy. The existing research yields inconsistent information on the associations between self-reported symptoms of psychological distress, such as depressive symptoms, during pregnancy, and HCC axis functioning. In a systematic review, Mustonen et al. (2018) reviewed studies assessing the link between maternal psychological distress and HCC; two of five studies reported a positive association between maternal prenatal psychological distress and HCC, and three reported no association. Mustonen et al. (2018) concluded that HCC appeared to be inconsistently associated with self-reported symptoms of prenatal psychological distress, especially in the range of mild-to-moderate symptom levels, and that characteristics of the study population and study design might partly explain whether associations between distress and HCC were observed. Self-reports of psychological distress usually cover short time periods (e.g., the last 1–2 weeks) that may not be closely related to HCC. Furthermore, all of the studies in the systematic review (Mustonen et al. 2018) included subjects from the general population with overall low levels of psychological distress and no data on psychiatric diagnoses.
A recent meta-analysis by Khoury et al. (2023) supplement these findings. Though the overall meta-analysis showed a non-significant effect between psychological distress and HCC, Khoury and colleagues (2023) report that studies using self-reported stress and depression showed significant associations between psychological distress and HPA activity. However, when stress was further subcategorized, only studies that assessed more chronic life stressors and not those that measured perceived stress or pregnancy stress were significantly associated with HPA axis functioning. In addition, moderator analyses indicated that the strength of the association between psychological distress and HCC was moderated by timing of HCC and distress measurement, so that effects were larger when distress was measured before HCC.
It seems that duration of stress more than certain types of psychological distress, related to mood, may be more strongly linked to HPA activity compared to more transient stressors. A Norwegian study assessing depressive symptoms during pregnancy did not find current symptoms to be associated with HCC in a community sample (Wikenius et al. 2016). They suggested that the construct of lifetime diagnosis might be more informative when evaluating the associations between HCC and psychopathology. Thus, including measures reflecting both long-term symptomatology as well as short-term psychological distress may be especially valuable in studies evaluating the association between maternal psychopathology and prenatal maternal HPA axis functioning.
The aim of this study is to assess psychopathology as a predictor of HCC in the 3rd trimester in pregnant women with severe mental disorders and non-clinical controls. We hypothesize that HCC is higher in individuals with a lifetime diagnosis of severe mental disorder compared to controls and that higher HCC is associated with ratings of poorer current symptomatic functioning based on a structured clinical interview.
Materials and methods
Participants
The study sample comprised N = 93 participants from the WARM study (Harder et al. 2015), of which 69 provided hair samples at pregnancy (n = 32 with severe mental illness (SMI), n = 37 non-clinical controls). Ethical approval for the WARM study came from Health Research Ethics, Capital Region of Denmark (protocol no: H-2-014-024). The study was preregistered in ClinicalTrials.gov Protocol Record DFF-1319-00103 (NCT02306551). Participants were recruited from obstetric wards in Region of Southern Denmark, the Capital Region, and Region Zealand, Denmark. Inclusion criteria were (1) women with first or subsequent pregnancies at the minimum age of 18 and (2) a diagnosis of either (a) lifetime DSM-5 delusional disorder, schizophreniform disorder, schizophrenia, schizoaffective disorder, psychosis NOS, and brief psychotic disorder, or (b) lifetime DSM-5 dipolar I or II disorder, or (c) a DSM-5 current or recurrent moderate-to-severe major depressive disorder, or (d) non-clinical controls. Exclusion criteria were (a) inability to provide informed and written consent to participate, (b) inability to speak English or Danish, (c) miscarriage, (d) maternal diagnosis of autism spectrum disorder, and (e) alcohol or drug dependency being the primary diagnosis. Further exclusion criteria were women describing current psychiatric symptoms not previously identified or treated and likely to require treatment.
Procedure
Sociodemographic and clinical assessments were collected during 2nd or 3rd trimester shortly after recruitment. Maternal hair was collected in the 3rd trimester. For further information on study design, see Harder et al. (2015).
Measures
Socio-demographics
Information on age, ethnicity, highest level of education, and civil status was self-reported. Education was categorized based on the ISCED-1997. All participants self-reported as married/living with a partner, or never married/not living with a partner, and civil status was dichotomized into “married or co-habiting”/not.
Clinical assessments
Psychopathology was measured by lifetime diagnosis of severe mental illness and current symptomatic functioning. Psychiatric diagnoses were confirmed using the psychosis and mood modules of the structured clinical interview (SCID) from the DSM-5 (First et al. 2016). All diagnostic assessments were supervised by a researcher trained on the SCID (KR, SH), and all diagnoses were confirmed through consensus discussion among the senior researchers. Based on the clinical interview, current symptomatic functioning was rated by the researcher using the symptomatic functioning subscale (GAF-S) of the global assessment of functioning scale (Niv et al. 2007). GAF is a numeric scale (0 through 100) with higher scores indicating better functioning. A score of 0 indicates insufficient information for rating, scores ranging from 10 to 20 indicate dangerous levels of symptoms present, and scores of 21–50 indicate dysfunctional levels of symptoms present. Scores of 51–70 indicate mild symptoms and some difficulty in functioning to serious symptoms and gross impairment in functioning. Scores above 71 indicate minimal symptoms and no more than slight impairment in functioning to no symptoms and superior functioning. Inter-rater reliability for the GAF-S subscale was acceptable (ICC (1) = 0.602).
Hair cortisol analyses
Hair samples were collected to determine cortisol concentrations. A section of hair strands approximately 3 mm in diameter was cut as close to the scalp as possible from a posterior vertex position. Hair samples were stored in aluminum foil as previously described by Anna-Hernandez et al. (2011) and analyzed at Dresden LabService GmbH, Germany, led by Prof. Dr. rer. nat. Clemens Kirschbaum, using liquid chromatography–tandem mass spectrometry (Gao et al. 2013). The 4-cm hair segment closest to the scalp was used for analyses to represent cortisol secretion over the most recent 4-month period (Stalder and Kirschbaum 2012). HCC values were standardized to the weight of the respective hair sample.
Confounding variables
Factors previously associated with maternal hair cortisol (Sauvé et al. 2007; Bublitz and Stroud 2012; Stalder and Kirschbaum 2012; Schreier et al. 2015; Marteinsdottir et al. 2021) were assessed as potential confounds, including (1) parity, (2) smoking, (3) regular use of medication, (4) body mass index (BMI), (5) waistline, (6) hair treatments over the past 3 months (dyeing, bleaching, permanent waves), (7) adverse childhood experiences, and (8) infant gender. Parity was categorized (yes/no) for primipara based on maternal self-report. Smoking was self-reported as number of cigarettes smoked in the past 7 days and categorized yes/no to smoking. Medication profile was self-reported as current use of any type of medication and categorized (yes/no). BMI was calculated by dividing weight (kg) by height squared (meter). Waistline was measured at the time of hair collection and reported in full cm. Use of chemical hair treatments over the past 3 months was self-reported at assessment and categorized (yes/no). Adverse childhood experiences were assessed using the self-report Adverse Childhood Experiences Study Questionnaire (ACES) (Felitti et al. 1998). The ACES has excellent internal reliability (Cronbach’s α = 0.88) (Murphy et al. 2013). Total number of adverse childhood experiences (ACE) was used as a continuous scale (range 0–10).
Data analytic plan
All analyses were conducted using IBM SPSS Statistics 28. HCCs were normally distributed after Log10 transformation, and ANOVAs were used to model unadjusted differences on HCC between the SMI and control group. Skewness of the main variables symptomatic functioning (GAF-S) and square root transformed ACE were within the assumption of the rule of + 1 to − 1 (Auyeung et al. 2009). Skewness of demographic and confounding variables, including education, maternal age, marital status, primipara, smoking, BMI, medication use, waistline, hair treatment within the past 3 months, and gestational age at cortisol collection, did not improve with transformations. Non-parametric Kruskal–Wallis test was used on skewed continuous variables. Pearson’s χ2 was used for dichotomized variables, and Fisher’s exact test 2-sided significance was reported. Non-parametric Spearman’s correlations of main variables, demographic variables, and all confounders were performed to identify covariates in subsequent analyses. Variables correlating significantly with HCC were controlled for in subsequent regression models that were run to estimate the effect of SMI and GAF-S score on HCC. All categorical variables entered into the regression model were dummy coded; i.e., infant gender was coded 1 for boys with girls as reference, parity was coded 1 for primiparity and 0 for multiparity, smoking was coded 1 and 0 for not smoking, and having a lifetime diagnosis of severe mental illness was coded 1 and non-clinical controls 0.
Results
Sample description and identification of control variables
Table 1 presents sample characteristics by groups of SMI and controls. The total sample presented as low on socio-economic risks, and most women from both groups were married or living with a partner. There were significant differences between the two groups on education level, age, and total ACE in unadjusted analyses. Women with SMI differed from women in the control group on the GAF-S. Mean GAF-S score among women in the control group was 87.86, with no participants scoring below 70 (one participant scored 70), indicating absence of symptoms and good functioning. Mean GAF-S score among women with SMI was 68.06, indicating some mild symptoms and some difficulties in functioning. Kruskal–Wallis and Chi-square analysis showed that there were no significant differences between participants who did and did not provide hair samples on any of the main variables or demographic measures.
Analysis of potential confounding variables, including (1) parity, (2) smoking, (3) regular use of medication, (4) BMI, (5) waistline, (6) hair treatments over the past 3 months, (7) total number of ACEs, and (8) infant gender, indicated that parity (r = 0.263, p = 0.030), education (r = − 0.286, p = 0.027), BMI (r = 0.268, p = 0.028), and smoking (r = 0.268, p = 0.042) were significantly related to maternal HCC in pregnancy. These significant variables were included as control variables in subsequent multivariate regression models.
Lifetime diagnosis of SMI as predictor of HCC
Unadjusted univariate ANOVA showed that HCC was significantly higher in women with SMI compared to controls (F(1) = 12.78, p = < 0.001) (Table 1). A multivariate regression model was run to assess the effect of SMI on HCC, controlling for the relevant control variables (Table 2). Results showed that the association between SMI and HCC was significant with a small effect size (β = 0.221) when controlling for parity, education, BMI, and smoking (Table 2).
Symptomatic functioning (GAF-S) as predictor of HCC
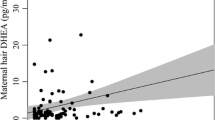
There was a significant correlation between GAF-S score and HCC (rs (67) = − 0.415, p = < 0.001). To assess the robustness of this finding in relation to potential confounds, a multivariate regression was run with parity, education, BMI, and smoking included as control variables (Table 3). Results showed that the association between GAF-S and HCC remained significant with a small effect size (β = − 0.011) (Table 3). As in the model with SMI, the control variable parity was significantly associated with HCC in this model with a medium effect size (β = 0.228) (Table 3).
Discussion
We hypothesized that severe psychopathology, conceptualized as a lifetime diagnosis of SMI and current symptomatology, was associated with higher HCC. Our findings showed that both lifetime diagnosis of SMI and current symptomatology were significantly associated with HCC. In addition, parity accounted for significant variance in HCC levels.
This seems to be in accordance with previous findings. In their meta-analysis, Khoury et al. (2023) showed that the timing of maternal HCC and psychological distress measurements moderated the effects, such that studies that measured distress before HCC had significantly larger effects, compared to studies that measured HCC before distress, or distress and HCC concurrently. This could support our finding of a significant effect of lifetime diagnosis on HCC, as all participants in the SMI group in our study had been diagnosed prior to inclusion in the study.
We also found that poorer current symptomatic functioning was significantly associated with elevated HCC in pregnancy. This seems to contradict previous findings, as women in our sample did not present with high levels of current symptoms; i.e., mean GAF-S rating among women from the SMI group indicated some mild symptoms and some difficulties in functioning. Previous studies have reported no association between current prenatal symptoms and HCC in participants with overall low levels of psychological distress (Mustonen et al. 2018). In the large FinnBrain cohort sample (N = 595), Mustonen et al. (2019) found that the trajectories of depressive symptoms in pregnancy were significant in relation to HPA axis functioning. Mustonen found that the subgroup of mothers with “low and steeply increasing” depressive symptoms (low in early and midpregnancy, but high in end pregnancy) did not show elevated HCC postpartum. However, HCC measured at delivery was elevated in relation to continuously higher levels of depressive symptoms, and the mothers in the “consistently elevated” group presented with higher HCC than mothers in both the “consistently low” and the “low and increasing” groups (Mustonen et al. 2019). This could support the hypothesis that chronicity of the prenatal symptoms, rather than short-term symptomatology, is relevant when HPA axis functioning is investigated. Momentary or short-term states of stress might not significantly affect the long-term biological measures such as HCC. In turn, altered functioning of the HPA axis could also contribute to the persistence of psychological distress or symptoms. In our study, women describing current psychiatric symptoms not previously identified or treated was excluded. In addition, only women from the SMI group presented with ratings of clinical to sub-clinical levels of symptomatic functioning on the GAF-S compared to the control group. It could be argued that the effect of current symptomatic functioning on HCC found in this sample is an effect of current symptomatology in concomitant of a lifetime diagnosis of severe mental illness. Thus, our sample may represent women who have had more chronic exposure to psychological distress over a lifetime, and a more continuous strain on the HPA axis, compared to women in the non-clinical control group, although some women from the SMI group did not present with high levels of current symptoms at the time of inclusion.
Studies reporting a positive association between prenatal psychological distress and HCC appear to have a larger variance in psychological distress symptom severity (Mustonen et al. 2018). Thus, our findings of significant effects of the constructs of lifetime diagnosis as well as current symptomatology on HCC in pregnancy may be partly explained by our sample characteristics including greater variance in psychiatric symptoms and higher frequency of lifetime distress symptoms.
Finally, short-term stress might not significantly affect the offspring risks related to prenatal psychological distress. Thus, identifying situations with different risk profiles would enable further understanding of the mechanisms linking prenatal maternal psychological distress to adverse offspring outcomes (Mustonen et al. 2018). In our study, both lifetime diagnosis of moderate-to-severe depression, bipolar disorder, or schizophrenia spectrum disorders and current symptomatic functioning predicted maternal HCC in late pregnancy and remained significant with other potential confounding variables controlled. This raises compelling concerns for the risks to both mother and infant of heightened glucocorticoids during this sensitive period of early development. In a previous study using a smaller subsample of the same participants from the WARM study, we found that maternal HCC in pregnancy and at 4 months postpartum mediated associations between maternal psychopathology and maternal disrupted behavior in interaction with the infant, both when psychopathology was measured as lifetime diagnosis and as current symptomatic functioning (Nyström-Hansen et al. 2019). These findings indicate that HCC may be a potential early biomarker for future caregiving challenges. Despite the difficulties in recruiting samples of women with severe mental illnesses during the limited timespan of pregnancy, our results clearly indicate the value of future studies with larger sample sizes to assess the replicability of the results reported here.
Limitations
Some limitations of the study should be noted. First, the small sample size limits the power to analyze potential differences between diagnostic groups. Second, this study did not include measures of other stressful life events or frequency of hair washing, which could affect HCC (Stalder et al. 2017). In addition, it could be noted that our measure of hair treatments (dyeing, bleaching, permanent waves) only asked about the past 3 months, though segments of 4 cm hair were analyzed reflecting the prior 4 months. However, in the recent study by Mustonen et al. (2019) on 595 participants from the large FinnBrain Birth Cohort Study, the researchers did not find HCC to be associated with hair-related attributes such as hair dying or hair washing. Similarly, meta-analytic estimates (Stalder et al. 2017) concerning the influences of hair washing frequency, and hair treatment suggested that although it is good practice to adjust for these factors, under most circumstances their influences would be practically negligible. Accordingly, we did not find a correlation between HCC and hair treatment.
Conclusions
Having a diagnosis of schizophrenia spectrum disorder, bipolar disorder, or current/recurrent moderate-to-severe major depressive disorder at some point prior to becoming pregnant predicts elevated maternal HCC during pregnancy. In addition, poorer current symptomatic functioning is associated with elevated HCC in pregnancy, compared to controls. The implications of elevated HCC on both maternal and infant health need further study. Hair cortisol concentration may be a potential early biomarker for developmental risk.
References
Andersen MS, Jensen RC, Schmedes AV, Brandslund I, Kyhl HB, Jensen TK, Glintborg D (2019) Third trimester cortisol status is associated with offspring sex and polycystic ovary syndrome status: Odense Child Cohort. Fertil Steril 112(4):0015–0282
Andreasen JJ, Tobiasen BB, Jensen RC, Boye H, Jensen TK, Bilenberg N, Andersen MS, Glintborg D (2023) Maternal cortisol in 3rd trimester is associated with traits of neurodevelopmental disorder in offspring. Odense Child Cohort Psychoneuroendocrinology 154:106293. https://doi.org/10.1016/j.psyneuen.2023.106293
Anna-Hernandez K, Ross RG, Natvig CL, Laudenslager ML (2011) Hair cortisol levels as a retrospective marker of hypothalamic–pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiol Behav 104:348–353
Auyeung B, Baron-Cohen S, Ashwin E, Knickmeyer R, Taylor K, Hackett G, Hines M (2009) Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. Psychol Sci 20(2):144–148
Bifulco A, Moran PM, Ball C, Jacobs C, Baines R, Bunn A, Cavagin J (2002) Childhood adversity, parental vulnerability and disorder: examining inter-generational transmission of risk. J Child Psychol Psychiatry 43:1075–1086
Borges S, Gayer-Anderson C, Mondelli V (2013) A systematic review of the activity of the hypothalamic-pituitary-adrenal axis in first episode psychosis. Psychoneuroendocrinology 38(5):603–611
Broeks CW, Choenni V, Kok R, van der Voorn B, de Kruijff I, van den Akker ELT, van Rossum EFC, Hoogendijk WJG, Hillegers MHJ, Kamperman AM, Lambregtse-Van den Berg MP (2021) An exploratory study of perinatal hair cortisol concentrations in mother-infant dyads with severe psychiatric disorders versus healthy controls. Bjpsych Open 7(1):e28. https://doi.org/10.1192/bjo.2020.159
Bublitz MH, Stroud LR (2012) Childhood sexual abuse is associated with cortisol awakening response over pregnancy: preliminary findings. Psychoneuroendocrinology 37(9):1425–1430
De Weerth C, Buitelaar JK (2005) Physiological stress reactivity in human pregnancy—a review. Neurosci Biobehav Rev 29:295–312
Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS (1998) Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) Study. Am J Prev Med 14(4):245–258
First MB, Williams JBW, Karg RS, Spitzer RL (2016) Structured clinical interview for DSM-5 disorders - clinical version (SCID-5-CV). American Psychiatric Association, Arlington, VA
Gao W, Stalder T, Foley P, Rauh M, Deng H, Kirschbaum C (2013) Quantitative analysis of steroid hormones in human hair using a column-switching LC-APCI-MS/MS assay. J Chromatogr B Anal Technol Biomed Life Sci 928:1–8
Harder S, Davidsen K, Macbeth A, Lange T, Minnis H, Andersen MS, Simonsen S, Lundy JM, Nyström-Hansen M, Trier CH, Roehder K, Gumley A (2015) Wellbeing and resilience: mechanisms of transmission of health and risk in parents with complex mental health problems and their offspring - the WARM study. BMC Psychiatry 15:310
Jung C, Ho JT, Torpy DJ, Rogers A, Doogue M, Lewis JG, Czajko RJ, Inder WJ (2011) A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab 96(5):1533–1540
Khoury JE, Giles L, Kaur H, Johnson D, Gonzalez A, Atkinson L (2023) Associations between psychological distress and hair cortisol during pregnancy and the early postpartum: a meta-analysis. Psychoneuroendocrinology 147:105969
Kirschbaum C, Tietze A, Skoluda N, Dettenborn L (2009) Hair as a retrospective calendar of cortisol production – Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology 34:32–37
Liu CH, Keshavan MS, Tronick E, Seidman LJ (2015) Perinatal risks and childhood premorbid indicators of later psychosis: next steps for early psychosocial interventions. Schizophr Bull 41(4):801–816. https://doi.org/10.1093/schbul/sbv047
Marteinsdottir I, Sydsjö G, Faresjö A, Theodorsson E, Josefsson A (2021) Parity-related variation in cortisol concentrations in hair during pregnancy. BJOG 128:637–644
Murphy A, Steele M, Dube SR, Bate J, Bonuck K, Meissner P, Goldman H, Steele H (2013) Adverse childhood experiences (ACEs) questionnaire and adult attachment interview (AAI): implications for parent child relationships. Child Abus Negl 38(2):224–233
Mustonen P, Karlssona L, Scheinina NM, Kortesluomaa S, Coimbrad B, Rodriguesd AJ, Karlsson H (2018) Hair cortisol concentration (HCC) as a measure for prenatal psychological distress — a systematic review. Psychoneuroendocrinology 92:21–28
Mustonen P, Karlssona L, Katajaa EL, Scheinina NM, Kortesluomaa S, Coimbrae B, Rodriguese AJ, Sousae N, Karlsson H (2019) Maternal prenatal hair cortisol is associated with prenatal depressive symptom trajectories. Psychoneuroendocrinology 109:104383
Niv N, Cohen AN, Sullivan G, Young AS (2007) The MIRECC version of the Global assessment of functioning scale: reliability and validity. Psychiatr Serv 58(4):529–535
Nyström-Hansen M, Andersen MS, Khoury JE, Davidsen K, Gumley A, Lyons-Ruth K, MacBeth A, Harder S (2019) Hair cortisol in the perinatal period mediates associations between maternal adversity and disrupted maternal interaction in early infancy. Dev Psychobiol 61(4):543–556. https://doi.org/10.1002/dev.21833
Rasic D, Hajek T, Alda M, Uher R (2013) Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: a meta-analysis of family high-risk studies. Schizophr Bull 40. https://doi.org/10.1093/schbul/sbt114
Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum SHM (2007) Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Investig Med 30:183–191
Schreier HMC, Enlow MB, Ritz T, Gennings C, Wright RJ (2015) Childhood abuse is associated with increased hair cortisol levels among urban pregnant women. J Epidemiol Community Health 69:1169–1174
Seckl J, Holmes M (2007) Mechanisms of Disease: glucocorticoids, their placental metabolism and fetal 'programming' of adult pathophysiology. Nat Rev Endocrinol 3, 479–488. https://doi.org/10.1038/ncpendmet0515
Stalder T, Kirschbaum C (2012) Analysis of cortisol in hair - state of the art and future directions. Brain Behav Immun 26(7):1019–1029
Stalder T, Steudte-schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, Kirschbaum C, Miller R (2017) Stress-related and basic determinants of hair cortisol in humans : a meta-analysis. Psychoneuroendocrinology 77:261–274
Staufenbiel SM, Penninx BWJH, Spijker AT, Elzinga BM, van Rossum EFC (2013) Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology 38:1220–1235
Uher R, Cumby J, MacKenzie LE, Morash-Conway J, Glover JM, Aylott A et al (2014) A familial risk enriched cohort as a platform for testing early interventions to prevent severe mental illness. BMC Psychiatry 14(1):344
Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, Hoyer D, Roseboom T, Räikkönen K, King S, Schwab M (2020) Prenatal developmental origins of behavior and mental health: the influence of maternal stress in pregnancy. Neurosci Biobehav Rev 117:26–64. https://doi.org/10.1016/j.neubiorev.2017.07.003
Wikenius E, Moe V, Kjellevold M, Smith L, Lyle R, Waagb R, Page CM, Myhre AM (2016) The association between hair cortisol and self-reported symptoms of depression in pregnant women. PLoS ONE 11(9):1–10
Willcutt E, McQueen M (2010) Genetic and environmental vulnerability to bipolar spectrum disorder. In: Miklowitz DJ, Cicchetti D (2010) (Eds) Understanding bipolar disorder: a developmental psychopathology perspective. New York: Guildford Press, pp 225–59
Zijlmans MAC, Marianne Riksen-Walraven J, de Weerth C (2015) Associations between maternal prenatal cortisol concentrations and child outcomes: a systematic review. Neurosci Biobehav Rev 53:1–24, ISSN 0149–7634. https://doi.org/10.1016/j.neubiorev.2015.02.015
Acknowledgements
We thank all the participating pregnant women for their contribution to this study, Rikke Agner Carstensen for her assistance in the data collection process, and the obstetric wards in Region Zealand, Region Southern Denmark, and the Capital Region in Denmark and Psychiatry, Region Zealand, for referring participants.
Funding
Open access funding provided by Copenhagen University The WARM study (Harder et al. 2015) received funding from the Danish Council for Independent Research/Humanities (Grant Reference No.: DFF–1319–00103); the Psychiatric Research Foundation in the Region of Southern Denmark; the Health Foundation of Region Zealand; and the NHS Research Scotland (NRS), through NHS Greater Glasgow & Clyde (NHSGG&C) and of the Scottish Mental Health Research Network (SMHRN). The funding sources did not play any role in the collection, analysis, or interpretation of the data.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Maja Nyström-Hansen, Kirstine Davidsen, Katrine Roehder, Christopher Trier, Emilie Nayberg, and Susanne Harder. The first draft of the manuscript was written by Maja Nyström-Hansen, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study sample comprised participants from the WARM study. Ethical approval for the WARM study came from Health Research Ethics, Capital Region of Denmark (Protocol No: H-2-014-024). The study was preregistered in ClinicalTrials.gov Protocol Record DFF-1319-00103 (NCT02306551). This study was performed in line with the principles of the Declaration of Helsinki. Written informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Christopher Trier is affiliated to private practice and Emilie Nayberg is affiliated to the District Psychiatry, Psychiatry East, Denmark.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nyström-Hansen, M., Andersen, M.S., Davidsen, K. et al. Hair cortisol concentrations in pregnant women with bipolar, depressive, or schizophrenic spectrum disorders. Arch Womens Ment Health (2024). https://doi.org/10.1007/s00737-024-01434-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00737-024-01434-4