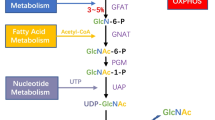
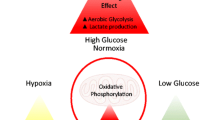
Abstract
O-linked β-N-actylglucosamine (O-GlcNAc) is a carbohydrate post-translational modification on hydroxyl groups of serine and/or threonine residues of cytosolic and nuclear proteins. Analogous to phosphorylation, O-GlcNAcylation plays crucial regulatory roles in a variety of cellular processes. O-GlcNAc was termed a nutritional sensor, as global levels of the modification are elevated in response to increased glucose and glutamine flux into the hexosamine biosynthetic pathway. A unique feature of cancer cell energy metabolism is a shift from oxidative phosphorylation to the less efficient glycolytic pathway (Warburg effect), necessitating greatly increased glucose uptake. Additionally, to help meet increased biosynthetic demands, cancer cells also up-regulate glutamine uptake. This led us to hypothesize that the universal feature of increased glucose and glutamine uptake by cancer cells might be linked to increased O-GlcNAc levels. Indeed, recent work in many different cancer types now indicates that hyper-O-GlcNAcylation is a general feature of cancer and contributes to transformed phenotypes. In this review, we describe known/potential links between hyper-O-GlcNAcylation and specific hallmarks of cancer, including cancer cell proliferation, survival, cell stresses, invasion and metastasis, aneuploidy, and energy metabolism. We also discuss inhibition of hyper-O-GlcNAcylation as a potential novel therapeutic target for cancer treatment.



Similar content being viewed by others
Abbreviations
- HBP:
-
Hexosamine biosynthetic pathway
- GlcNAc:
-
N-acetylglucosamine
- PDAC:
-
Pancreatic ductal adenocarcinoma
- OGA:
-
O-GlcNAcase
- HPDE:
-
Human pancreatic duct epithelial cell
- PPP:
-
Pentose phosphate pathway
- HSR:
-
Heat shock response
- EMT:
-
Epithelial to mesenchymal transition
References
Alison MR, Lin WR, Lim SM, Nicholson LJ (2012) Cancer stem cells: in the line of fire. Cancer Treat Rev 38(6):589–598. doi:10.1016/j.ctrv.2012.03.003
Andres-Bergos J, Tardio L, Larranaga-Vera A, Gomez R, Herrero-Beaumont G, Largo R (2012) The increase in O-linked N-acetylglucosamine protein modification stimulates chondrogenic differentiation both in vitro and in vivo. J Biol Chem 287(40):33615–33628. doi:10.1074/jbc.M112.354241
Badet B, Vermoote P, Haumont PY, Lederer F, LeGoffic F (1987) Glucosamine synthetase from Escherichia coli: purification, properties, and glutamine-utilizing site location. Biochemistry 26(7):1940–1948
Baeriswyl V, Christofori G (2009) The angiogenic switch in carcinogenesis. Semin Cancer Biol 19(5):329–337. doi:10.1016/j.semcancer.2009.05.003
Balch WE, Morimoto RI, Dillin A, Kelly JW (2008) Adapting proteostasis for disease intervention. Science 319(5865):916–919. doi:10.1126/science.1141448
Bang D, Wilson W, Ryan M, Yeh JJ, Baldwin AS (2013) GSK-3alpha promotes oncogenic KRAS function in pancreatic cancer via TAK1-TAB stabilization and regulation of noncanonical NF-κB. Cancer discov 3(6):690–703. doi:10.1158/2159-8290.cd-12-0541
Benz CC, Yau C (2008) Ageing, oxidative stress and cancer: paradigms in parallax. Nat Rev Cancer 8(11):875–879. doi:10.1038/nrc2522
Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D (2000) Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2(10):737–744. doi:10.1038/35036374
Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S, Vosseller K, Reginato MJ (2010) Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene 29(19):2831–2842. doi:10.1038/onc.2010.41
Chou TY, Dang CV, Hart GW (1995a) Glycosylation of the c-Myc transactivation domain. Proc Natl Acad Sci U S A 92(10):4417–4421
Chou TY, Hart GW, Dang CV (1995b) c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. J Biol Chem 270(32):18961–18965
Dai C, Whitesell L, Rogers AB, Lindquist S (2007) Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell 130(6):1005–1018. doi:10.1016/j.cell.2007.07.020
Dai C, Dai S, Cao J (2012) Proteotoxic stress of cancer: implication of the heat-shock response in oncogenesis. J Cell Physiol 227(8):2982–2987. doi:10.1002/jcp.24017
DeBerardinis RJ (2008) Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet Med 10(11):767–777. doi:10.1097/GIM.0b013e31818b0d9b
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7(1):11–20. doi:10.1016/j.cmet.2007.10.002
Dudeja V, Chugh RK, Sangwan V, Skube SJ, Mujumdar NR, Antonoff MB, Dawra RK, Vickers SM, Saluja AK (2011) Prosurvival role of heat shock factor 1 in the pathogenesis of pancreatobiliary tumors. Am J Phys Gastrointest Liver Physiol 300(6):G948–G955. doi:10.1152/ajpgi.00346.2010
Earhart RH, Amato DJ, Chang AY, Borden EC, Shiraki M, Dowd ME, Comis RL, Davis TE, Smith TJ (1990) Phase II trial of 6-diazo-5-oxo-L-norleucine versus aclacinomycin-A in advanced sarcomas and mesotheliomas. Invest New Drugs 8(1):113–119
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3(5):362–374. doi:10.1038/nrc1075
Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV (2009) c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458(7239):762–765. doi:10.1038/nature07823
Gloster TM, Zandberg WF, Heinonen JE, Shen DL, Deng L, Vocadlo DJ (2011) Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within cells. Nat Chem Biol 7(3):174–181. doi:10.1038/nchembio.520
Gong J, Jing L (2011) Glutamine induces heat shock protein 70 expression via O-GlcNAc modification and subsequent increased expression and transcriptional activity of heat shock factor-1. Minerva Anestesiol 77(5):488–495
Gordon DJ, Resio B, Pellman D (2012) Causes and consequences of aneuploidy in cancer. Nat Rev Genet 13(3):189–203. doi:10.1038/nrg3123
Gregory MA, Qi Y, Hann SR (2003) Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J Biol Chem 278(51):51606–51612. doi:10.1074/jbc.M310722200
Gross BJ, Kraybill BC, Walker S (2005) Discovery of O-GlcNAc transferase inhibitors. J Am Chem Soc 127(42):14588–14589. doi:10.1021/ja0555217
Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C, Yang J, Han F, Lu X, Yu W (2010) GlcNAcylation plays an essential role in breast cancer metastasis. Cancer Res 70(15):6344–6351. doi:10.1158/0008-5472.can-09-1887
Guinez C, Lemoine J, Michalski JC, Lefebvre T (2004) 70-kDa-heat shock protein presents an adjustable lectinic activity towards O-linked N-acetylglucosamine. Biochem Biophys Res Commun 319(1):21–26. doi:10.1016/j.bbrc.2004.04.144
Guinez C, Filhoulaud G, Rayah-Benhamed F, Marmier S, Dubuquoy C, Dentin R, Moldes M, Burnol AF, Yang X, Lefebvre T, Girard J, Postic C (2011) O-GlcNAcylation increases ChREBP protein content and transcriptional activity in the liver. Diabetes 60(5):1399–1413. doi:10.2337/db10-0452
Gupta GP, Massague J (2006) Cancer metastasis: building a framework. Cell 127(4):679–695. doi:10.1016/j.cell.2006.11.001
Hallor KH, Sciot R, Staaf J, Heidenblad M, Rydholm A, Bauer HC, Astrom K, Domanski HA, Meis JM, Kindblom LG, Panagopoulos I, Mandahl N, Mertens F (2009) Two genetic pathways, t(1;10) and amplification of 3p11-12, in myxoinflammatory fibroblastic sarcoma, haemosiderotic fibrolipomatous tumour, and morphologically similar lesions. J Pathol 217(5):716–727. doi:10.1002/path.2513
Haltiwanger RS, Blomberg MA, Hart GW (1992) Glycosylation of nuclear and cytoplasmic proteins. Purification and characterization of a uridine diphospho-N-acetylglucosamine:polypeptide beta-N-acetylglucosaminyltransferase. J Biol Chem 267(13):9005–9013
Hanahan D, Folkman J (1996) Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86(3):353–364
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674. doi:10.1016/j.cell.2011.02.013
Hart GW (1997) Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annu Rev Biochem 66:315–335. doi:10.1146/annurev.biochem.66.1.315
Hart GW, Housley MP, Slawson C (2007) Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446(7139):1017–1022. doi:10.1038/nature05815
Havula E, Hietakangas V (2012) Glucose sensing by ChREBP/MondoA-Mlx transcription factors. Semin Cell Dev Biol 23(6):640–647. doi:10.1016/j.semcdb.2012.02.007
Ho SR, Wang K, Whisenhunt TR, Huang P, Zhu X, Kudlow JE, Paterson AJ (2010) O-GlcNAcylation enhances FOXO4 transcriptional regulation in response to stress. FEBS Lett 584(1):49–54. doi:10.1016/j.febslet.2009.11.059
Itkonen HM, Minner S, Guldvik IJ, Sandmann MJ, Tsourlakis MC, Berge V, Svindland A, Schlomm T, Mills IG (2013) O-GlcNAc transferase integrates metabolic pathways to regulate the stability of c-MYC in human prostate cancer. Cancer Res. doi:10.1158/0008-5472.can-13-0549
Jang H, Kim TW, Yoon S, Choi SY, Kang TW, Kim SY, Kwon YW, Cho EJ, Youn HD (2012) O-GlcNAc regulates pluripotency and reprogramming by directly acting on core components of the pluripotency network. Cell Stem Cell 11(1):62–74. doi:10.1016/j.stem.2012.03.001
Kawauchi K, Araki K, Tobiume K, Tanaka N (2008) p53 regulates glucose metabolism through an IKK-NF-kappaB pathway and inhibits cell transformation. Nat Cell Biol 10(5):611–618. doi:10.1038/ncb1724
Kawauchi K, Araki K, Tobiume K, Tanaka N (2009) Loss of p53 enhances catalytic activity of IKKbeta through O-linked beta-N-acetyl glucosamine modification. Proc Natl Acad Sci U S A 106(9):3431–3436. doi:10.1073/pnas.0813210106
Kazemi Z, Chang H, Haserodt S, McKen C, Zachara NE (2010) O-linked beta-N-acetylglucosamine (O-GlcNAc) regulates stress-induced heat shock protein expression in a GSK-3beta-dependent manner. J Biol Chem 285(50):39096–39107. doi:10.1074/jbc.M110.131102
Kisner DL, Catane R, Muggia FM (1980) The rediscovery of DON (6-diazo-5-oxo-L-norleucine). Recent Results Cancer Res 74:258–263
Kreppel LK, Hart GW (1999) Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J Biol Chem 274(45):32015–32022
Kroemer G, Pouyssegur J (2008) Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13(6):472–482. doi:10.1016/j.ccr.2008.05.005
Lazarus MB, Nam Y, Jiang J, Sliz P, Walker S (2011) Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature 469(7331):564–567. doi:10.1038/nature09638
Liu J, Marchase RB, Chatham JC (2007) Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J Mol Cell Cardiol 42(1):177–185. doi:10.1016/j.yjmcc.2006.09.015
Livingston RB, Venditti JM, Cooney DA, Carter SK (1970) Glutamine antagonists in chemotherapy. Adv Pharmacol Chemother 8:57–120
Luo J, Solimini NL, Elledge SJ (2009) Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 136(5):823–837. doi:10.1016/j.cell.2009.02.024
Lynch G, Kemeny N, Casper E (1982) Phase II evaluation of DON (6-diazo-5-oxo-L-norleucine) in patients with advanced colorectal carcinoma. Am J Clin Oncol 5(5):541–543
Lynch TP, Ferrer CM, Jackson SR, Shahriari KS, Vosseller K, Reginato MJ (2012) Critical role of O-Linked β-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. J Biol Chem 287(14):11070–11081. doi:10.1074/jbc.M111.302547
Ma Z, Vocadlo DJ, Vosseller K (2013) Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-κB activity in pancreatic cancer cells. J Biol Chem 288(21):15121–15130. doi:10.1074/jbc.M113.470047
Manzari B, Kudlow JE, Fardin P, Merello E, Ottaviano C, Puppo M, Eva A, Varesio L (2007) Induction of macrophage glutamine: fructose-6-phosphate amidotransferase expression by hypoxia and by picolinic acid. Intern J Immunopathol Pharmacol 20(1):47–58
Mariappa D, Sauert K, Marino K, Turnock D, Webster R, van Aalten DM, Ferguson MA, Muller HA (2011) Protein O-GlcNAcylation is required for fibroblast growth factor signaling in Drosophila. Sci Signal 4 (204): ra89. doi:10.1126/scisignal.2002335
Marotta NP, Cherwien CA, Abeywardana T, Pratt MR (2012) O-GlcNAc modification prevents peptide-dependent acceleration of α-synuclein aggregation. Chembiochem. doi:10.1002/cbic.201200478
Martindale JL, Holbrook NJ (2002) Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 192(1):1–15. doi:10.1002/jcp.10119
McGranahan N, Burrell RA, Endesfelder D, Novelli MR, Swanton C (2012) Cancer chromosomal instability: therapeutic and diagnostic challenges. EMBO Rep 13(6):528–538. doi:10.1038/embor.2012.61
Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X, Cong Q, Yu W (2011) O-GlcNAcylation is a novel regulator of lung and colon cancer malignancy. Biochim Biophys Acta 1812(4):514–519. doi:10.1016/j.bbadis.2011.01.009
Moore EC, Lepage GA (1957) In vivo sensitivity of normal and neoplastic mouse tissues to azaserine. Cancer Res 17(8):804–808
Moreno-Sanchez R, Rodriguez-Enriquez S, Marin-Hernandez A, Saavedra E (2007) Energy metabolism in tumor cells. FEBS J 274(6):1393–1418. doi:10.1111/j.1742-4658.2007.05686.x
Mueller MM, Fusenig NE (2004) Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 4(11):839–849. doi:10.1038/nrc1477
Myatt SS, Lam EW (2007) The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer 7(11):847–859. doi:10.1038/nrc2223
Myers SA, Panning B, Burlingame AL (2011) Polycomb repressive complex 2 is necessary for the normal site-specific O-GlcNAc distribution in mouse embryonic stem cells. Proc Natl Acad Sci U S A 108(23):9490–9495. doi:10.1073/pnas.1019289108
Nandi A, Sprung R, Barma DK, Zhao Y, Kim SC, Falck JR (2006) Global identification of O-GlcNAc-modified proteins. Anal Chem 78(2):452–458. doi:10.1021/ac051207j
Neckers L, Workman P (2012) Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res 18(1):64–76. doi:10.1158/1078-0432.ccr-11-1000
Ngoh GA, Watson LJ, Facundo HT, Jones SP (2011) Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids 40(3):895–911. doi:10.1007/s00726-010-0728-7
O’Donnell N, Zachara NE, Hart GW, Marth JD (2004) Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol Cell Biol 24(4):1680–1690
Ohn T, Kedersha N, Hickman T, Tisdale S, Anderson P (2008) A functional RNAi screen links O-GlcNAc modification of ribosomal proteins to stress granule and processing body assembly. Nat Cell Biol 10(10):1224–1231. doi:10.1038/ncb1783
Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M, Xu Y, Wonsey D, Lee LA, Dang CV (2000) Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem 275(29):21797–21800. doi:10.1074/jbc.C000023200
Ovejera AA, Houchens DP, Catane R, Sheridan MA, Muggia FM (1979) Efficacy of 6-diazo-5-oxo-L-norleucine and N-[N-gamma-glutamyl-6-diazo-5-oxo-norleucinyl]-6-diazo-5-oxo-norleucine against experimental tumors in conventional and nude mice. Cancer Res 39(8):3220–3224
Park SY, Kim HS, Kim NH, Ji S, Cha SY, Kang JG, Ota I, Shimada K, Konishi N, Nam HW, Hong SW, Yang WH, Roth J, Yook JI, Cho JW (2010a) Snail1 is stabilized by O-GlcNAc modification in hyperglycaemic condition. EMBO J. doi:10.1038/emboj.2010.254
Park SY, Kim HS, Kim NH, Ji S, Cha SY, Kang JG, Ota I, Shimada K, Konishi N, Nam HW, Hong SW, Yang WH, Roth J, Yook JI, Cho JW (2010b) Snail1 is stabilized by O-GlcNAc modification in hyperglycaemic condition. EMBO J 29(22):3787–3796. doi:10.1038/emboj.2010.254
Pathak S, Borodkin VS, Albarbarawi O, Campbell DG, Ibrahim A, van Aalten DM (2012) O-GlcNAcylation of TAB 1 modulates TAK1-mediated cytokine release. EMBO J 31(6):1394–1404. doi:10.1038/emboj.2012.8
Peinado H, Olmeda D, Cano A (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7(6):415–428. doi:10.1038/nrc2131
Perkins ND (2012) The diverse and complex roles of NF-kappaB subunits in cancer. Nat Rev Cancer 12(2):121–132. doi:10.1038/nrc3204
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radical Biol Med 49(11):1603–1616. doi:10.1016/j.freeradbiomed.2010.09.006
Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ, Kincead-Beal C, Kulkarni P, Varambally S, Ghosh D, Chinnaiyan AM (2007) Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 9(2):166–180
Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM (2004) Oncomine: a cancer microarray database and integrated data-mining platform. Neoplasia 6(1):1–6
Roos MD, Han IO, Paterson AJ, Kudlow JE (1996) Role of glucosamine synthesis in the stimulation of TGF-alpha gene transcription by glucose and EGF. Am J Physiol 270(3 Pt 1):C803–C811
Rozanski W, Krzeslak A, Forma E, Brys M, Blewniewski M, Wozniak P, Lipinski M (2012) Prediction of bladder cancer based on urinary content of MGEA5 and OGT mRNA level. Clin Lab 58(5–6):579–583
Sakiyama H, Fujiwara N, Noguchi T, Eguchi H, Yoshihara D, Uyeda K, Suzuki K (2010) The role of O-linked GlcNAc modification on the glucose response of ChREBP. Biochem Biophys Res Commun 402(4):784–789. doi:10.1016/j.bbrc.2010.10.113
Shi Y, Tomic J, Wen F, Shaha S, Bahlo A, Harrison R, Dennis JW, Williams R, Gross BJ, Walker S, Zuccolo J, Deans JP, Hart GW, Spaner DE (2010) Aberrant O-GlcNAcylation characterizes chronic lymphocytic leukemia. Leukemia 24(9):1588–1598. doi:10.1038/leu.2010.152
Singleton KD, Wischmeyer PE (2008) Glutamine induces heat shock protein expression via O-glycosylation and phosphorylation of HSF-1 and Sp1. JPEN J Parenter Enteral Nutr 32(4):371–376. doi:10.1177/0148607108320661
Slawson C, Zachara NE, Vosseller K, Cheung WD, Lane MD, Hart GW (2005) Perturbations in O-linked beta-N-acetylglucosamine protein modification cause severe defects in mitotic progression and cytokinesis. J Biol Chem 280(38):32944–32956. doi:10.1074/jbc.M503396200
Sola-Penna M, Da Silva D, Coelho WS, Marinho-Carvalho MM, Zancan P (2010) Regulation of mammalian muscle type 6-phosphofructo-1-kinase and its implication for the control of the metabolism. IUBMB Life 62(11):791–796. doi:10.1002/iub.393
Tarnowski GS, Stock CC (1957) Effects of combinations of azaserine and of 6-diazo-5-oxo-L-norleucine with purine analogs and other antimetabolites on the growth of two mouse mammary carcinomas. Cancer Res 17(10):1033–1039
Teo CF, Ingale S, Wolfert MA, Elsayed GA, Not LG, Chatham JC, Wells L, Boons GJ (2010) Glycopeptide-specific monoclonal antibodies suggest new roles for O-GlcNAc. Nat Chem Biol 6(5):338–343. doi:10.1038/nchembio.338
Thiery JP, Sleeman JP (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 7(2):131–142. doi:10.1038/nrm1835
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–890. doi:10.1016/j.cell.2009.11.007
Tomic J, McCaw L, Li Y, Hough MR, Ben-David Y, Moffat J, Spaner DE (2013) Resveratrol has anti-leukemic activity associated with decreased O-Glcnacylated proteins. Exp Hematol. doi:10.1016/j.exphem.2013.04.004
Tong X, Zhao F, Mancuso A, Gruber JJ, Thompson CB (2009) The glucose-responsive transcription factor ChREBP contributes to glucose-dependent anabolic synthesis and cell proliferation. Proc Natl Acad Sci U S A 106(51):21660–21665. doi:10.1073/pnas.0911316106
Torres CR, Hart GW (1984) Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem 259(5):3308–3317
Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147(2):275–292. doi:10.1016/j.cell.2011.09.024
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324(5930):1029–1033. doi:10.1126/science.1160809
Vervoorts J, Luscher-Firzlaff J, Luscher B (2006) The ins and outs of MYC regulation by posttranslational mechanisms. J Biol Chem 281(46):34725–34729. doi:10.1074/jbc.R600017200
Vierbuchen T, Wernig M (2012) Molecular roadblocks for cellular reprogramming. Mol Cell 47(6):827–838. doi:10.1016/j.molcel.2012.09.008
Walgren JL, Vincent TS, Schey KL, Buse MG (2003) High glucose and insulin promote O-GlcNAc modification of proteins, including alpha-tubulin. Am J Physiol Endocrinol Metabol 284(2):E424–E434. doi:10.1152/ajpendo.00382.2002
Wang Z, Udeshi ND, Slawson C, Compton PD, Sakabe K, Cheung WD, Shabanowitz J, Hunt DF, Hart GW (2010) Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates cytokinesis. Sci Signal 3 (104):ra2. doi:10.1126/scisignal.2000526
Warburg O (1956a) On respiratory impairment in cancer cells. Science 124(3215):269–270
Warburg O (1956b) On the origin of cancer cells. Science 123(3191):309–314
Warburg O, Wind F, Negelein E (1927) The metabolism of tumors in the body. J General Physiol 8(6):519–530
Ward PS, Thompson CB (2012) Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 21(3):297–308. doi:10.1016/j.ccr.2012.02.014
Weis SM, Cheresh DA (2011) Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 17(11):1359–1370. doi:10.1038/nm.2537
Wells L, Vosseller K, Hart GW (2001) Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science 291(5512):2376–2378
Wells L, Vosseller K, Hart GW (2003) A role for N-acetylglucosamine as a nutrient sensor and mediator of insulin resistance. Cell Mol Life Sci 60(2):222–228
Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB (2008) Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A 105(48):18782–18787. doi:10.1073/pnas.0810199105
Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP (2009) Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell 15(5):416–428. doi:10.1016/j.ccr.2009.03.016
Xia Y, Rocchi P, Iovanna JL, Peng L (2012) Targeting heat shock response pathways to treat pancreatic cancer. Drug Discov Today 17(1–2):35–43. doi:10.1016/j.drudis.2011.09.016
Yehezkel G, Cohen L, Kliger A, Manor E, Khalaila I (2012) O-GlcNAcylation in primary and metastatic colorectal cancer clones and effect of O-GlcNAcase silencing on cell phenotype and transcriptome. J Biol Chem. doi:10.1074/jbc.M112.345546
Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA 3rd, Peters EC, Driggers EM, Hsieh-Wilson LC (2012) Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science 337(6097):975–980. doi:10.1126/science.1222278
Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL, Yan H, Wang W, Chen S, Viale A, Zheng H, Paik JH, Lim C, Guimaraes AR, Martin ES, Chang J, Hezel AF, Perry SR, Hu J, Gan B, Xiao Y, Asara JM, Weissleder R, Wang YA, Chin L, Cantley LC, DePinho RA (2012) Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149(3):656–670. doi:10.1016/j.cell.2012.01.058
Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y (2007) Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol 178(1):93–105. doi:10.1083/jcb.200703099
Yuzwa SA, Shan X, Macauley MS, Clark T, Skorobogatko Y, Vosseller K, Vocadlo DJ (2012) Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nat Chem Biol 8(4):393–399. doi:10.1038/nchembio.797
Zachara NE, Hart GW (2004) O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophy Acta 1673(1–2):13–28
Zachara NE, O’Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW (2004) Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem 279(29):30133–30142. doi:10.1074/jbc.M403773200
Zhu W, Leber B, Andrews DW (2001) Cytoplasmic O-glycosylation prevents cell surface transport of E-cadherin during apoptosis. EMBO J 20(21):5999–6007. doi:10.1093/emboj/20.21.5999
Zhu Q, Zhou L, Yang Z, Lai M, Xie H, Wu L, Xing C, Zhang F, Zheng S (2012) O-GlcNAcylation plays a role in tumor recurrence of hepatocellular carcinoma following liver transplantation. Med Oncol 29(2):985–993. doi:10.1007/s12032-011-9912-1
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, Z., Vosseller, K. O-GlcNAc in cancer biology. Amino Acids 45, 719–733 (2013). https://doi.org/10.1007/s00726-013-1543-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-013-1543-8