Abstract
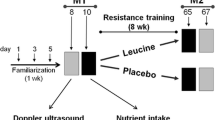
An increase in the capacity of athletic performance depends on adequate nutrition, which ensures optimal function of the musculoskeletal system, including tendon stability. However, little is known about the status of tendons and extracellular matrix modifications during malnutrition and nutritional recovery when leucine is used in response to exercise conditioning. The purpose of this study was to evaluate the collagen content and biomechanical aspects of the deep digital flexor tendon (DDFT) in malnourished rats submitted to nutritional recovery (control diet or leucine-rich diet) and aerobic physical activity. After 60 days of undernourishment (6% protein diet), the malnourished rats were subsequently nutritionally recovered with a control diet or leucine-rich diet and trained or not (swimming, without overload) for 5 weeks. The biomechanical analysis and quantification of hydroxyproline were assessed in the DDFT in all experimental groups. The leucine-rich diet increased hydroxyproline content in the tension region, independently of the training. In the compression region, hydroxyproline content was higher in the malnourished and leucine-trained groups. Biomechanical analysis showed a lower load in the malnourished and all-trained groups. The lowest stress was observed with control-trained animals. The nutritional-recovered groups showed higher strain values corresponding to control group, while the lowest values were observed in malnourished and trained groups. The results suggest that a leucine-rich diet stimulates collagen synthesis of the DDFT, especially when in combination with physical exercise, and seems to determine the increase of resistance and the biomechanical characteristics of tendons.




Similar content being viewed by others
References
Aro AA, Tomiosso TC, Vidal BC, Gomes L, Matiello-Rosa SMG, Pimentel ER (2008) Structural and biochemical analysis of the effect o immobilization followed by stretching on the calcaneal tendon of rats. Connect Tissue Res 49:443–454
Bailey AJ (2001) Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev 122:735–755
Balage M, Dardevet D (2010) Long-term effects of leucine supplementation on body composition. Curr Opin Clin Nutr Metab Care 13:265–270
Banos CC, Thomas AH, Kuo CK (2008) Collagen fibrillogenesis in tendon development: current models and regulation of fibril assembly. Birth Defects Res (Part C) 84:228–244
Benevides GP, Pimentel ER, Toyama MH et al (2004) Biochemical and biomechanical analysis of tendon of caged and penned chickens. Connect Tissue Res 45:206–215
Benjamin M, McGonagle D (2009) Entheses: tendon and ligament attachment sites. Scand J Med Sci Sports 19:520–527
Bianchi G, Marzocchi R, Agostini F et al (2005) Update on nutritional supplementation with branched-chain amino acids. Curr Opin Clin Nutr Metab Care 8:83–87
Brabaj JA, Cuthbertson DJR, Smith K et al (2005) Collagen synthesis in human musculoskeletal tissues and skin. Am J Physiol Endocrinol Metab 289:E864–E869
Buchanan CI, Marsh RL (2002) Effects of exercise on the biomechanical, biochemical and structural properties of tendons. Comp Biochem Physiol Part A 133:1101–1107
Canty EG, Kadler KE (2002) Collagen fibril biosynthesis in tendon: a review and recent insights. Comp Biochem Physiol Part A 133:979–985
Enwemeka CS, Maxwell LC, Fernandes G (1992) Ultrastructural morphometry of metrical changes induced by exercise and food restriction in the rat calcaneal tendon. Tissue Cell 24:499–510
Fessel G, Snedeker JG (2010) Equivalent stiffness after glycosaminoglycan depletion in tendon—an ultra-structural finite element model and corresponding experiments. J Theor Biol. doi:10.1016/j.jtbi.2010.10.007
Gad SC, Weil CS (1994) Statistic for toxicologists. In: Wallace H (ed) Principles and methods of toxicology. Raven Press, New York, pp 221–274
Hutson SM, Harris RA (2001) Leucine as a nutritional signal. J Nutr 131:839S–840S
Izu Y, Ansorge HL, Zhang G, Soslowsky LJ, Bonaldo P, Chu ML, Birk DE (2010) Dysfunctional tendon collagen fibrillogenesis in collagen VI null mice. Matrix Biol. doi:10.1016/j.matbio.2010.10.001
Kilts T, Ameye L, Syed-Picard F, Ono M, Berendsen AD, Oldberg A, Heegaard AM, Bi Y, Young MF (2009) Potential roles for the small leucine-rich proteoglycans biglycan and fibromodulin in ectopic ossification of tendon induced by exercise and in modulating rotarod performance. Scand J Med Sci Sports 19(4):536–546
Kjaer M (2004) Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84:649–698
Kjær M, Langberg H, Heinemeier K, Bayer ML, Hansen M, Holm L, Doessing S, Kongsgaard M, Krogsgaard MR, Magnusson SP (2009) From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports 19(4):500–510
Layman DK (2003) The role of leucine in weight loss diets and glucose homeostasis. J Nutr 133:261S–267S
Lehnert SA, Byrne KA, Reverter A, Nattrass GS et al (2006) Gene expression profiling of bovine skeletal muscle in response to and during recovery from chronic and severe undernutrition. J Anim Sci 84(12):3239–3250
Lin TW, Cardenas L, Soslowsky LJ (2003) Biomechanics of tendon injury and repair. J Biomech 37:865–877
MacKenna D, Summerrour SR, Villarreal FJ (2000) Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc Res 46:257–263
Magnusson SP, Hansen P, Kjae M (2003) Tendon properties in relation to muscular activity and physical training. Scand J Med Sports 13:211–223
Millward DJ (2003) An adaptative metabolic demand model for protein and amino acids requirements. Br J Nutr 90:249–260
Natale VM, Brenner IK, Moldoneanu AI et al (2003) Effects of three different types of exercise on blood leukocyte count during and following exercise. Sao Paulo Med J 121:9–14
Nicastro H, Artioli GG, Costa AS, Solis MY, Luz CL, Blachier F, Lancha AH (2010) An overview of the therapeutic effects of leucine supplementation on skeletal muscle under atrophic conditions. Amino Acids. doi:10.1007/s00726-010-0636-x
Nosaka K, Clarkson PM (1996) Changes in indicators of inflammation after eccentric exercise of the elbow flexors. Med Sci Sports Exerc 28:953–961
O’Brien M (1997) Structure and metabolism of tendons. Scand J Med Sci Sports 7:55–61
Oxlund H, Andreassen TT (1992) Aminoguanidine treatment reduces the increase in collagen stability of rats with experimental diabetes mellitus. Diabetologia 35(1):19–25
Pike AVL, Ker RF, Alexander RMN (2000) The development of fatigue quality in high and low stressed tendons of sheep (Ovis aries). J Exp Biol 203:2187–2193
Reed CC, Iozzo RV (2002) The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J 19:249–255
Rees SG, Dent CM (2009) Caterson B.Metabolism of proteoglycans in tendon. Scand J Med Sci Sports 19(4):470–478
Reeves PG, Nielsen FH, Fahey GC (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Reiser KM (1994) Influence of age and long-term dietary restriction on enzymatically mediated crosslinks and nonenzymatic glycation of collagen in mice. J Geront 49:B71–B79
Rennie MJ (2007) Exercise- and nutrient-controlled mechanisms involved in maintenance of the musculoskeletal mass. Biochem Soc Trans 35(Pt 5):1302–1305
Schechtman H, Bader DL (1997) In vitro fatigue of human tendons. J Biomech 30:829–835
Simonsen EB, Kligaard H, Bojsen-Moller F (1995) The influence of strength training, swim training and ageing on the Achilles tendon and muscle soleus of the rat. J Sports Sci 13:291–295
Smith K, Rennie MJ (2007) New approaches and recent results concerning human-tissue collagen synthesis. Curr Opin Clin Nutr Metab Care 10(5):582–590
Stegemann H, Stalder K (1967) Determination of hydroxyproline. Clin Chim Acta 18:267–273
Svensson L, Aszodi A, Reinholt FP et al (1999) Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem 274:9636–9647
Tsuzaki M, Yamauchi M, Banes AJ (1993) Tendon collagens: extracellular matrix composition in shear stress and tensile components of flexor tendons. Connect Tissue Res 29:141–152
Ventrucci G, Silva LGR, Mello MAR, Gomes-Marcondes MCC (2004) Effects os a leucine-rich diet on body composition during nutritional recovery in rats. Nutrition 20:213–217
Vidal BC, Carvalho HF (1990) Aggregational state and molecular order of tendons as a function of age. Matrix 10:48–57
Wayburn B, Volk T (2009) LRT, a tendon-specific leucine-rich repeat protein, promotes muscle-tendon targeting through its interaction with Robo. Development 136(21):3607–3615
Yoon JH, Brooks R, Kim YH et al (2003) Proteoglycans in chicken gastrocnemius tendons change with exercise. Arch Biochem Biophys 412:279–286
Zanchi NE, Gerlinger-Romero F, Guimaraes-Ferreira L, Filho MAS, Felitti V, Lira S, Seelaender M, Lancha AH (2010) HMB supplementation: clinical and athletic performance-related effects and mechanisms of action. Amino Acids. doi:10.1007/s00726-010-0678-0
Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S, Birk DE (2005) Development of tendon structure and function: regulation of collagen fibrillogenesis. J Musculoskelet Neuronal Interact 5(1):5–21
Acknowledgments
Financial support was provided by Fapesp (#04/00514-5, #01/2135-3) and CNPq (#304000/2007-8; # 502915/2007-2. The authors thank Dr. Maria Alice Rostom de Mello for reviewing the English grammar and Dr. Juvenal Marcondes Neto for statistical support. Carbohydrate and dextrin were donated by Corn Products (Sao Paulo, Brazil), and amino acids were donated by Ajinomoto Brasil (Sao Paulo, Brazil). The manuscript was edited by native English speaking editors at American Journal Experts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barbosa, A.W.C., Benevides, G.P., Alferes, L.M.T. et al. A leucine-rich diet and exercise affect the biomechanical characteristics of the digital flexor tendon in rats after nutritional recovery. Amino Acids 42, 329–336 (2012). https://doi.org/10.1007/s00726-010-0810-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0810-1