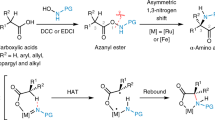
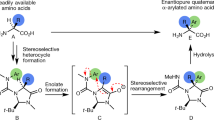
Abstract
An atom-efficient and stereoselective synthesis has been developed for the preparation of α-2H-labelled (S)-α-amino acids, starting from a novel chiral diketopiperazine scaffold. Efficient mono-alkylation of the chiral template afforded the (S)-substituted adducts with the nature of the electrophile significantly effecting the stereochemical outcome. Subsequent alkylation was totally selective producing the 1,4-cis adduct as the sole diastereoisomer. The deprotection was carried out using cerium ammonium nitrate followed by acid hydrolysis affording the enantipure α-amino acids.


Similar content being viewed by others
References
Bachmann S, Knudsen KR, Jorgensen KA (2004) Mimicking enzymatic transaminations: attempts to understand and develop a catalytic asymmetric approach to chiral α-amino acids. Org Biomol Chem 2:2044–2049
Balducci D, Lazzari I, Monari M, Piccinelli F, Porzi G (2009a) (S)-α-methyl, α-amino acids: a new stereocontrolled synthesis. Amino Acids. doi:10.1007/s00726-009-0289-9
Balducci D, Contaldi S, Lazzari I, Porzi G (2009b) A highly efficient stereocontrolled synthesis of (S)-2′,63/3/2010-dimethyltyrosine [(S)-DMT]. Tetrahedron Asymmetr 20:1398–1401
Bull SD, Davies SG, O’Shea MD (1998a) Stereoselective conjugate addition of organocuprates to a dehydroalanine derived diketopiperazine. J Chem Soc Perkin Trans 1(1):3657–3658
Bull SD, Davies, SG, Epstein SW, Ouzman VA (1998b) Chiral relay auxiliary for the synthesis of enantiomerically pure a-amino acids. Chem Commun 659–660
Bull SD, Davies SG, Garner AC, Parkes AL, Roberts PM, Sellers TGR, Smith AD, Tamayo JA, Thomson JE, Vickers RJ (2007) On the origins of diastereocelectivity in the alkylation of diketopiperazine enolates. New J Chem 31:486–495
Davies SG, Garner AC, Ouzman VA, Roberts PM, Smith AD, Snow EJ, Thomson JE, Tamayo JA, Vickers RJ (2007) Diastereoselective synthesis of quaternary α-amino acids from diketopiperazine templates. Org Biomol Chem 5:2138–2147
Ducho C, Hamed RB, Batchelar ET, Sorensen JL, Odell B, Schofield CJ (2009) Synthesis of regio- and stereoselectively deuterium-labelled derivatives of l-glutamate semialdehyde for studies on carbapenem biosynthesis. Org Biomol Chem 7:2770–2779
Felpin FX, Doris E, Wagner A, Valleix A, Rousseau B, Mioskowski C (2000) Rearrangement of α-amino cyclopropanone hydrate: a novel route to labeled amino acids. J Org Chem 66:305–308
Ferioli F, Piccinelli F, Porzi G, Sandri S (2002) Stereoselective synthesis of bis(α-amino acid) derivatives isosteric with cysteine. Part 4. Tetrahedron Asymmetr 13:1181
Gout E, Chesne S, Beguin CG, Pelmont J (1978) Kinetic studies with the use of Proton-Magnetic-Resonance Spectroscopy of the specific α-deuteration of amino acids by Escherichia coli aspartate aminotransferase. Biochem J 171:719–723
Holding AN, Spencer JB (2008) Investigation into the mechanism of phenolic couplings during the biosynthesis of glycopeptide antibiotics. ChemBioChem 9:2209–2214
Moran GR, Derecskei-Kovacs A, Hillas PJ, Fitzpatrick PF (2000) On the catalytic mechanism of tryptophan hydroxylase. J Am Chem Soc 122:4535–4541
Nakanishi T, Miyazawa M, Sakakura M, Terasawa H, Takahashi H, Shimada I (2002) Determination of the interface of a large protein complex by transferred cross-saturation measurements. J Mol Biol 318:245–249
O’Reilly E, Lestini E, Balducci D, Paradisi F (2009) One-step diketopiperazine synthesis using phase transfer catalysis. Tetrahedron Lett 50:1748–1750
O’Reilly E, Pes L, Paradisi F (2010) From amines to diketopiperazines: a one-pot approach. Tetrahedron Lett 51:1696–1697
Oba M, Terauchi T, Owari Y, Imai Y, Motoyamab I, Nishiyama K (1998) Stereo-divergent synthesis of l-threo- and l-erythro-[2, 3–2H2]amino acids using optically active dioxopiperazine as a chiral template. J Chem Soc Perkin Trans 1:1275–1281
Oba M, Iwasaki A, Hitokawa H, Ikegame T, Banba H, Ura K, Takamura T, Nishiyama K (2006) Preparation of l-serine and l-cystine stereospecifically labeled with deuterium at the β-position. Tetrahedron Asymmetr 17:1890–1894
Ong S-E, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M (2002) Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics 1:376–386
Orena M, Porzi G, Sandri S (1992) Diastereoselective alkylation of (3S)- and (3R)-3-methylpiperazine-2, 5-dione derivatives. A convenient approach to both (S)- and (R)-alanine. J Org Chem 57:6532
Orena M, Porzi G, Sandri S (1993) (3R)-Methylpiperazine-2 and (3S)-methylpiperazine-2,5-dione derivatives as useful intermediates in the enantioselective synthesis of alpha-amino esters. J Chem Res Synop 318
Paradisi F, Porzi G, Rinaldi S, Sandri S (2000a) A simple asymmetric synthesis of (+)- and (−)-2,6-diaminopimelic acids. Tetrahedron Asymmetr 11:1259
Paradisi F, Porzi G, Rinaldi S, Sandri S (2000b) Stereoselective synthesis of α, α′-diamino-dicarboxylic acids part 2. Tetrahedron Asymmetr 11:4617
Paradisi F, Piccinelli F, Porzi G, Sandri S (2002) Enantioselective synthesis of 2, 6-diaminopimelic acid derivatives. Part 3. Tetrahedron Asymmetr 13:497
Pearce DA, Sargeson AM, Hammershoi A, Harrowfield JM (2000) A simple regiospecific strategy for labelling hydrogen atoms in α-amino acids. Chem Commun 2431–2432
Piccinelli F, Porzi G, Sandri M, Sandri S (2003) Stereocontrolled synthesis of enantiomerically pure unsaturated analogues of 2, 6-DAP. Part 5. Tetrahedron Asymmetr 14:393
Porzi G, Sandri S (1994) Synthesis of (3R, 6R)- and (3S, 6S)-3, 6-dialkylpiperazin-2, 5-dione derivatives as useful intermediates to both (R) and (S) α-aminoacids. Tetrahedron Asymmetr 5:453–464
Rose J, Leeson P, Gani D (1995) Stereospecific synthesis of α-deuterated α-amino acids: regiospecific deuteration of chiral 3-isopropyl-2, 5-dimethoxy-3, 6-dihydropyrazines. J Chem Soc Perkin Trans 1:157–165
Sattler M, Fesik SW (1996) Use of deuterium labeling in NMR: overcoming a sizeable problem. Structure 4:1245–1249
Schöllkopf U, Groth U, Deng C (1891) Enantioselective syntheses of (R)-amino acids using L-valine as chiral agent. Angew Chem Int Ed Engl 20:798–799
Schöllkopf U, Hartwig W, Groth U (1979) Enantioselective synthesis of α-Methyl-α-aminocarboxylic acids by alkylation of the lactim ether of cyclo-(L-Ala-L-Ala). Angew Chem Int Ed Engl 18:863–864
Smith AD, Bull SD, Davies SG, Epstein SW, Garner AC, Mujtaba N, Tamayo JA, Watkin DJ (2006) Enantiodiscrimination of racemic electrophiles using diketopiperazine enolates: asymmetric synthesis of methyl 2–amino-3 aryl aspartates and 3-methyl-aspartates. Tetrahedron 62:7911–7925
Takeuchi K, Ng E, Malia T, Wagner G (2007) 1-13C amino acid selective labeling in a 2H 15 N background for NMR studies of large proteins. J Biomol NMR 38:89–98
Veenstra TD, Martinovic S, Anderson GA, Pasa-Tolic L, Smith RD (2000) Proteome analysis using selective incorporation of isotopically labeled amino acids. J Am Soc Mass Spectrom 11:78–82
Acknowledgments
We express our gratitude to Sustainable Energy Ireland, administered by the Irish Research Council for Science, Engineering and Technology (IRCSET) for funding Elaine O’Reilly. We would also like to acknowledge the facilities of the Centre for Synthesis and Chemical Biology (CSCB), funded by the Higher Education Authorities Programme for Research in Third-Level Institutions (PRTLIs). We are grateful to Prof. Patrick Guiry for the use of his Perkin-Elmer 241 polarimeter.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
O’Reilly, E., Balducci, D. & Paradisi, F. A stereoselective synthesis of α-deuterium-labelled (S)-α-amino acids. Amino Acids 39, 849–858 (2010). https://doi.org/10.1007/s00726-010-0541-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0541-3