Summary.
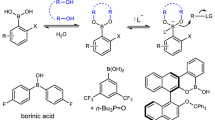
Acetophenone, 2,2-dimethylcyclopentanone, 3,3-dimethyl-2-butanone, 3-methyl-2-butanone, and 2-pentanone were reduced with borane mediated by (S)-alanine, (S)-methionine, (S)-leucine, (S)-valine, and (S)-isoleucine in very good yields giving predominantly alcohols of (R)-configuration (ee = 23–89%). A molecular topology based model was developed for describing the influence of the substituents, both in the oxazaborolidine type reagent and in the ketone, on the observed chiral induction.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received May 31, 2001. Accepted June 25, 2001
Rights and permissions
About this article
Cite this article
Teodorović, A., Joksović, M., Gutman, I. et al. Amino Acid Mediated Borane Reduction of Ketones IIa . Monatshefte für Chemie 133, 23–29 (2002). https://doi.org/10.1007/s007060270003
Issue Date:
DOI: https://doi.org/10.1007/s007060270003