Abstract
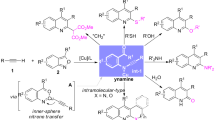
In this work, the reactivity of 2-nitro-5,10,15,20-tetraphenylporphyrin with 2-(4-nitrophenyl)acetonitrile in the presence of KOH as base was studied. Under these conditions, three new compounds were isolated: a β-di-substituted derivative, as the major compound, accompanied by two minor products, a π-extended and a β-isoxazoline-fused derivative, all in acceptable yields. A preliminary study was also performed in the presence of K2CO3. It allowed the isolation of a cyclopropyl-annulated chlorin in very good yield. All the obtained products were photochemically and photophysically characterized, some of them showing promising properties to be used as photosensitizers in photodynamic processes.
Graphical abstract




Similar content being viewed by others
References
Kadish KM, Smith KM, Guilard R (2010) Handbook of Porphyrin Science. World Scientific Publishing Company Co, Singapore
Di Carlo G, Biroli AO, Tessore F, Caramori S, Pizzotti M (2018) Coord Chem Rev 358:153
Kundu S, Patra A (2017) Chem Rev 117:712
Vamsi KN, Suman KJV, Madoori M, Seelam P, Lingamallu G (2017) Chemsuschem 10:4668
Nakagaki S, Mantovani K, Sippel Machado G, de Freitas Dias, Castro K, Wypych F (2016) Molecules 21:291
Costentin C, Robert M, Savéant J-M (2015) Acc Chem Res 48:2996
Pegis ML, Wise CF, Martin DJ, Mayer JM (2018) Chem Rev 118:2340
Zhang W, Lai W, Cao R (2017) Chem Rev 117:3717
Jurow M, Schuckman AE, Batteas JD, Drain CM (2010) Coord Chem Rev 254:2297
Niu T, Li A (2013) J Phys Chem Lett 4:4095
Auwärter W, Écija D, Klappenberger F, Barth JV (2015) Nat Chem 7:105
Lu H, Kobayashi N (2016) Chem Rev 116:6184
Otsuki J (2018) J Mater Chem A 6:6710
Paolesse R, Nardis S, Monti D, Stefanelli M, Di Natale C (2017) Chem Rev 117:2517
Ding Y, Zhu W-H, Xie Y (2017) Chem Rev 117:2203
Figueira F, Rodrigues JMM, Farinha AAS, Cavaleiro JAS, Tomé JPC (2016) J Porphyrins Phthalocyanines 20:950
Singh S, Aggarwal A, Bhupathiraju NVSDK, Arianna G, Tiwari K, Drain CM (2015) Chem Rev 115:10261
Calvete MJF, Pinto SMA, Pereira MM, Geraldes CFGC (2017) Coord Chem Rev 333:82
Zhou Y, Liang X, Dai Z (2016) Nanoscale 8:12394
Diogo P, Fernandes C, Caramelo F, Mota M, Miranda IM, Faustino MAF, Neves MGPMS, Uliana MP, Santos JM, Gonçalves T (2017) Front Microbiol 8:498
Marciel L, Teles L, Moreira B, Pacheco M, Lourenço LMO, Neves MGPMS, Tomé JPC, Faustino MAF, Almeida A (2017) Future. Med Chem 9:365
Cerqueira A, Moura NMM, Serra VIV, Faustino MAF, Tomé AC, Cavaleiro JAS, Neves MGPMS (2017) Molecules 22:1269
Cavaleiro JAS, Tomé AC, Neves MGPMS (2010) Meso-tetraarylporphyrin derivatives: New synthetic methodologies. In: Kadish KM, Smith KM, Guilard R (eds) Handbook of porphyrin science. World Scientific Publishing Company Co, Singapore, p 193
Serra VIV, Pires SMG, Alonso CMA, Neves MGPMS, Tomé AC, Cavaleiro JAS (2014) Meso-tetraarylporphyrins bearing nitro or amino groups: synthetic strategies and reactivity profiles. In: Paolesse R (ed) Synthesis and modifications of porphyrinoids. Springer, Berlin, p 35
Crossley MJ, King LG, Simpson JL (1997) J Chem Soc Perkin Trans 1 3087 and references herein cited
Jaquinod L (2000) Functionalization of 5,10,15,20-tetra-substituted porphyrins. In: Kadish KM, Smith KM, Guilard R (eds) The porphyrin handbook. Academic, San Diego, p 212
Jaquinod L, Gros C, Olmstead MM, Antolovich M, Smith KM (1996) Chem Commun 1475
Jaquinod L, Gros C, Khoury RG, Smith KM (1996) Chem Commun 2581
Luo D, Carter KA, Miranda D, Lovell JF (2017) Adv Sci 4:1600106
Ethirajan M, Chen Y, Joshi P, Pandey RK (2011) Chem Soc Rev 40:340
Abrahamse H, Hamblin MR (2016) Biochem J 473:347
Josefsen LB, Boyle RW (2012) Theranostics 2:916
Gomes ATPC, Neves MGPMS, Cavaleiro JAS (2018) An Acad Bras Ciênc 90:993
Alves E, Faustino MAF, Neves MGPMS, Cunha Â, Nadais H, Almeida A (2015) J Photochem Photobiol C 22:34
Tim M (2015) J Photochem Photobiol B 150:2
Rosa LP, Silva FC (2014) J Med Microb Diagn 3:158
Sperandio FF, Ying-Ying H, Hamblin MR (2013) Recent Pat Anti-Infect Drug Discov 8:108
Adolfo VDM, Haynes MH, Ball AB, Tianhong D, Christos A, Kelso MJ, Hamblin MR, Tegos GP (2012) Photochem Photobiol 88:499
Wainwright M, Maisch T, Nonell S, Plaetzer K, Almeida A, Tegos GP, Hamblin MR (2017) Lancet Infect Dis 17:e49
Marko AJ, Patel NJ, Joshi P, Missert JR, Pandey RK (2016) In: Pandey RK, Kessel D, Dougherty TJ (eds) Handbook of photodynamic therapy—updates on recent applications of porphyrin-based compounds. World Scientific Publishing Co, Singapore, p 3
van Straten D, Mashayekhi V, de Bruijn HS, Oliveira S, Robinson DJ (2017) Cancers 9:54
Baptista MS, Cadet J, Di Mascio P, Ghogare AA, Greer A, Hamblin MR, Lorente G, Nunez SC, Ribeiro MS, Thomas AH, Vignoni M, Yoshimura TM (2017) Photochem Photobiol 93:912
Yun SH, Kwok SJJ (2017) Nat Biomed Eng 1:16
Baldwin JE, Crossley MJ, DeBernardis J (1982) Tetrahedron 38:685
Alonso CMA, Neves MGPMS, Tomé AC, Silva AMS, Cavaleiro JAS (2005) Tetrahedron 61:11866
Faustino MA, Neves MGPMS, Vicente MGH, Silva AMS, Cavaleiro JS (1995) Tetrahedron Lett 36:5977
Batalha PN, Gomes ATPC, Forezi LSM, Costa L, de Souza MCBV, Boechat FdCS, Ferreira VF, Almeida A, Neves MGPMS, Cavaleiro JAS (2015) RSC Adv 5:71228
Bastos MM, Gomes ATPC, Neves MGPMS, Santos-Filho OA, Boechat N, Cavaleiro JAS (2013) Eur J Org Chem 1485
Hashimoto T, Choe Y-K, Nakano H, Hirao K (1999) J Phys Chem A 103:1894
Baskin JS, Yu H-Z, Zewail AH (2002) J Phys Chem A 106:9837
Durantini J, Otero L, Funes M, Durantini EN, Fungo F, Gervaldo M (2011) Electrochim Acta 56:4126
Maximiano RV, Piovesan E, Zílio SC, Machado AEH, de Paula R, Cavaleiro JAS, Borissevitch IE, Ito AS, Gonçalves PJ, Neto NMB (2010) J Photochem Photobiol A 214:115
Berlman IB (1971) Handbook of fluorescence spectra of aromatic molecules, 2nd edn. Academic, New York
Montalti AC, Prodi L, Gandolfi MT (2006) Handbook of photochemistryn, 3rd edn. Taylor & Francis, Boca Raton
Spiller W, Kliesch H, Wöhrle D, Hackbarth S, Röder B, Schnurpfeil G (1998) J Porphyrins Phthalocyanines 2:145
Zenkevich E, Sagun E, Knyukshto V, Shulga A, Mironov A, Efremova O, Bonnett R, Songca SP, Kassem M (1996) J Photochem Photobiol B 33:171
Armarego WLF, Perrin DD (1996) Purification of laboratory chemicals, 4th edn. Butterworth-Heinemann, Oxford
Ermilov EA, Tannert S, Werncke T, Choi MTM, Ng DKP, Röder B (2006) Chem Phys 328:428
Acknowledgements
Thanks are due to FCT/MEC for the financial support to the QOPNA research Unit (FCT UID/QUI/00062/2013), through national funds and when applicable co-financed by the FEDER, within the PT2020 Partnership Agreement and “Compete” 2020, and also to the Portuguese NMR Network. N. M. M. Moura thanks to FCT for his postdoctoral Grant (SFRH/BPD/84216/2012). The authors also thank the Transnational cooperation program, FCT-CNRST (Morocco), for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amiri, O., Moura, N.M.M., Faustino, M.A.F. et al. Synthetic access to new porphyrinoids from 2-nitro-5,10,15,20-tetraphenylporphyrin and an arylacetonitrile. Monatsh Chem 150, 67–75 (2019). https://doi.org/10.1007/s00706-018-2283-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-018-2283-y