Abstract
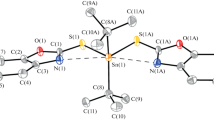
New square-planar Pd(II) and Pt(II) complexes with 8-quinolinecarboxaldehyde selenosemicarbazone have been synthesized and characterized by use of elemental analysis, molar conductivity measurements, and IR and NMR spectroscopy. The cytotoxic activity of the ligand, new Pt(II) and Pd(II) compounds, and previously synthesized Pd(II), Pt(II), Cd(II), and Ni(II) complexes with the analogous ligand, 2-quinolinecarboxaldehyde selenosemicarbazone, was tested against two human cancer cell lines: lung carcinoma (H460) and glioma (U251). The potential of these compounds to induce perturbations of the H460 cell cycle was also evaluated. These substances had an excellent radical-scavenging effect against ABTS radical cations. The best antimicrobial activity, among two yeasts and eight bacterial strains tested, was against Bacillus cereus.
Graphical Abstract





Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Lobana TS, Sharma R, Bawa G, Khanna S (2009) Coord Chem Rev 253:977
Casas JS, Garcia-Tasende MS, Sordo J (2000) Coord Chem Rev 209:197
West DX, Liberta AE, Padhye SB, Chikate RC, Sonawane PB, Kumbhar AS, Yerande RG (1993) Coord Chem Rev 123:49
West DX, Padhye SB, Sonawane PB (1991) Struct Bond 76:1
Pelosi G (2010) Open Crystallogr J 3:16
Schwarz K, Foltz CM (1957) J Am Chem Soc 79:3292
Castle TC, Maurer RI, Sowrey FE, Went MJ, Reynolds CA, McInnes EJL, Blower PJ (2003) J Am Chem Soc 125:10040
Todorović TR, Bacchi A, Pelizzi G, Juranić NO, Sladić DM, Brčeski I, Anđelković KK (2006) Inorg Chem Commun 9:862
Andaloussi MBD, Mohr FJ (2010) Organomet Chem 695:1276
Kowol CR, Eichinger R, Jakupec MA, Galanski M, Arion VB, Keppler BK (2007) J Inorg Biochem 101:1946
Pizzo C, Faral-Tello P, Salinas G, Flo M, Robello C, Wipfe P, Mahler SG (2012) Med Chem Comm 3:362
Turk SR, Shipman C Jr, Drach JC (1986) J Gen Virol 67:1625
Shipman C Jr, Smith SH, Drach JC, Klayman DL (1986) Antiviral Res 6:197
Mautner HG, Kumler WD, Okano Y, Pratt R (1956) Antibiot Chemother 6:51
Revenko MD, Prisacari VI, Dizdari AV, Stratulat EF, Corja ID, Proca LM (2011) Pharm Chem J 45:351
Agrawal KC, Booth BA, Michaud RL, Moore EC, Sartorelli AC (1974) Biochem Pharmacol 23:2421
Todorović TR, Bacchi A, Juranić NO, Sladić DM, Pelizzi G, Božić T, Filipović NR, Anđelković KK (2007) Polyhedron 26:3428
Todorović TR, Bacchi A, Sladić DM, Todorović NM, Božić T, Radanović DD, Filipović NR, Pelizzi G, Anđelković KK (2009) Inorg Chim Acta 362:3813
Gligorijević N, Todorović T, Radulović S, Sladić D, Filipović N, Gođevac D, Jeremić D, Anđelković K (2009) Eur J Med Chem 44:1623
Bjelogrlić S, Todorović T, Bacchi A, Zec M, Sladić D, Srdić-Rajić T, Radanović D, Radulović S, Pelizzi G, Anđelković K (2010) J Inorg Biochem 10:4673
Srdić-Rajić T, Zec M, Todorović T, Anđelković K, Radulović S (2011) Eur J Med Chem 46:3734
Zec M, Srdić-Rajić T, Konić-Ristić A, Todorović T, Anđelković K, Filipović-Ljesković I, Radulović S (2012) Anticancer Agents Med Chem 12:1071
Lallemand Y-I, Soulié J, Chottard J-C (1980) J Chem Soc Chem Commun 436(10)
Chiang SY, Welch J, Rauscher FJ, Beerman TA (1994) Biochemistry 33:7033
Sankaran M, Kumarasamy C, Chokkalingam U, Mohan PS (2010) Bioorg Med Chem Lett 20:7147
Malakyan MG, Badzhinyan SA, Vardevanyan LA, Grigoryan DS, Egiazaryan DE, Avetisyan AA, Aleksanyan IL, Ambartsumyan LP, Sargsyan KS (2009) Pharm Chem J 43:7
Battin EE, Brumaghim JL (2009) Cell Biochem Biophys 55:1
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Free Radic Biol Med 26:1231
Raičević N, Mladenović A, Perović M, Harhaji L, Miljković D, Trajković V (2005) Neuropharmacology 48:720
Kethcum PA (1988) Microbiology: concept and applications. Wiley, New York
Bowman FW (1969) J Pharm Sci 58:1301
Acknowledgments
This work was supported by the Ministry of Education, Science, and Technological Development of the Republic of Serbia (Grants OI 172055, III 41025, and III 46010).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material. 1H–15N HMBC spectra of ligand HL1 and complex 1; representative antimicrobial activity plots for the ligands, complexes 1–6, and standard antimicotic and antibiotics, and the appropriate solid and liquid media used for cultivation of microorganisms are given in the Electronic Supplementary Material.
Rights and permissions
About this article
Cite this article
Filipović, N., Polović, N., Rašković, B. et al. Biological activity of two isomeric N-heteroaromatic selenosemicarbazones and their metal complexes. Monatsh Chem 145, 1089–1099 (2014). https://doi.org/10.1007/s00706-014-1197-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-014-1197-6