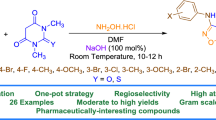
Abstract
Schiff-base Kabachnik–Fields intermediates generated in situ from substituted pyrazole-4-carbaldehyde and 2-aminothiophene derivatives were trapped by dialkyl phosphites to produce the corresponding α-aminophosphonates in moderate yields. The latter products could be also obtained in excellent yields (≥75%) by directly applying the phosphorus reagents to the Schiff bases. Next, dialkyl phosphites were applied to one of the parent aldehydes to give the expected α-hydroxyphosphonate derivatives. Applying hexaalkyl triamidophosphites to the Schiff base in ethanol afforded methylphosphonic diamide derivatives, whereas ring attack on the pyrazole ring occurred when the same amidophosphites were applied to the parent aldehyde to give the corresponding alkylidenephosphorane ylides in an open structure form in good yields. Some of the new compounds exhibited considerable anti-inflammatory properties.
Graphical abstract







Similar content being viewed by others
References
Quin LD (2000) A guide to organophosphorus chemistry. Wiley-Interscience, New York
Westheimer FH (1987) Science 235:1173
Kafarski P, Lejczak B (2001) Curr Med Chem 1:301
Bloemink MJ, Diederen JJH, Dorenbos JP, Heetebrij RJ, Keppler BK, Reedijik J (1999) Eur J Inorg Chem 10:1655
Rao X, Song Z, He L (2008) Heteroatom Chem 19:512
Makhaeva GF, Malygin VV, AYu Aksinenko, Sokolov VB, Strakhova NN, Rasdolsky AN, Richardson RJ, Martynov IV (2005) Dokl Biochem Biophys 400:92
Baylis EK, Campbell CD, Dingwall JG (1984) J Chem Soc Perkin Trans 1:2845
Abdou WM, Salem MAI, Barghash RF (2007) Arkivoc 15:45
Abdou WM, Sediek AA, Khidre MD (2008) Monatsh Chem 139:617
Abdou WM, Shaddy AM (2008) Lett Org Chem 5:569
Abdou WM, Khidre MD, Khidre RE (2009) Eur J Med Chem 44:526
Abdou WM, Shaddy AM, Sediek AA (2009) J Chem Res Synth
Abdou WM, Ganoub NA, Sabry E (2009) Z Naturforsch 46b:1057
Abdou WM, Khidre RE (2009) Monatsh Chem 141:219
Akbari J, Heydari A (2009) Tetrahedron Lett 50:4236
Hosseini-Sarvari M (2008) Tetrahedron 64:5459
Lu SM, Chen RY (2000) Heteroatom Chem 11:217
Kaboudin B, Nazari R (2001) Tetrahedron Lett 42:8211
Matveeva ED, Zefirov NS (2008) Dokl Chem 420:137
Kaboudin B (2002) Phosphorus, Sulfur Silicon Relat Elem 177:1749
Babudri F, Fiandanese V, Musio R, Naso F, Sciavovelli O, Scilimati A (1991) Synthesis 225
Scherowsky G, Weiland J (1974) Chem Ber 107:3155
Ramirez F, Ruhm D, Smith CP (1965) Tetrahedron 21:1941
Lucken EAC, Ramirez F, Catto VP, Ruhm D, Dershovitz S (1966) Tetrahedron 22:637
Ramirez F, Patwardhan AV, Kugler HJ, Smith CP (1967) J Am Chem Soc 89:6275
Hadjipavlou-Litina D (1993) Res Commun Chem Pathol Pharmacol 81:1091
Sounders BC, Stark BP (1958) Tetrahedron 4:197
Acknowledgments
Financial support from the Egyptian Academy of Scientific Research and Technology is gratefully acknowledged. We are grateful to the National Central Lab of Toxicology, Cairo, Egypt.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdou, W.M., Barghash, R.F. & Bekheit, M.S. Multicomponent reactions in a one-pot synthesis of α-aminophosphonates and α-aminophosphonic diamides with anti-inflammatory properties. Monatsh Chem 142, 649–656 (2011). https://doi.org/10.1007/s00706-011-0492-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-011-0492-8