Summary.
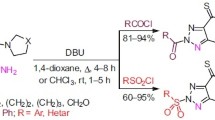
The preparation of N-acylsulfonamides is described using primary amines, arylsulfonyl chlorides and acyl chlorides. Reaction of primary aryl amines with arylsulfonyl chlorides in the presence of NaHCO3 produced N-arylsulfonamides, which reacted in situ with benzoyl chloride furnishing the corresponding N-benzoyl-N-arylsulfonamides in 72–96% yields. Accordingly, 4-nitrobenzoyl chloride and 3,5-dinitrobenzoyl chloride were used as acylating agents. All the reactions were carried out under solvent-free conditions at room temperature and the products were isolated after simple work-up in high yields and purity.
Similar content being viewed by others
References
T Hasegawa H Yamamoto (2000) Bull Chem Soc Jpn 73 423 Occurrence Handle10.1246/bcsj.73.423 Occurrence Handle1:CAS:528:DC%2BD3cXpsVSntA%3D%3D
MG Banwell CF Crasto CJ Easton AK Forrest T Karoli DR March L Mensah MR Nairn PJ O’Hanlon MD Oldham W Yue (2000) Bioorg Med Chem Lett 10 2263 Occurrence Handle10.1016/S0960-894X(00)00456-X Occurrence Handle1:CAS:528:DC%2BD3cXntF2lu7w%3D
Y Wang DL Soper MJ Dirr MA Delong B De JA Wos (2000) Chem Pharm Bull 48 1332 Occurrence Handle1:CAS:528:DC%2BD3cXmsVersr8%3D
LL Chang WT Ashton KL Flanagan TB Chen SS O’Malley GJ Zingaro PKS Siegl SD Kivlighn VJ Lotti RSL Chang WJ Greenlee (1994) J Med Chem 37 4464 Occurrence Handle10.1021/jm00052a006 Occurrence Handle1:CAS:528:DyaK2MXitFOgsbw%3D
JH Musser AF Kreft RHW Bender DM Kubrak D Grimes RP Carlson JM Hand J Chang (1990) J Med Chem 33 240 Occurrence Handle10.1021/jm00163a039 Occurrence Handle1:CAS:528:DyaK3cXjsFKmsw%3D%3D
K Kondo E Sekimoto J Nakao Y Murakami (2000) Tetrahedron 56 5843 Occurrence Handle10.1016/S0040-4020(00)00549-4 Occurrence Handle1:CAS:528:DC%2BD3cXlt12jtb8%3D
K Kondo E Sekimoto K Miki Y Murakami (1998) J Chem Soc Perkin Trans 1 2973 Occurrence Handle10.1039/a806186f
N Ishizuka KI Matsumura K Hayashi K Sakai T Yamamori (2000) Synthesis 6 784 Occurrence Handle10.1055/s-2000-6270
MT Martin F Roschangar JF Eaddy (2003) Tetrahedron Lett 44 5461 Occurrence Handle10.1016/S0040-4039(03)01324-8 Occurrence Handle1:CAS:528:DC%2BD3sXkvVGrsrY%3D
DU Singh PR Singh SD Samant (2004) Tetrahedron Lett 45 4805 Occurrence Handle10.1016/j.tetlet.2004.04.077 Occurrence Handle1:CAS:528:DC%2BD2cXkt1Wmtrk%3D
AR Katritzky S Hoffmann K Suzuki (2004) Arkivoc 12 14
DG Liu Y Gao JH Voigt K Lee MC Nicklaus L Wu ZY Zhang TR Bruke (2003) Bioorg Med Chem Lett 13 3005 Occurrence Handle10.1016/S0960-894X(03)00635-8 Occurrence Handle1:CAS:528:DC%2BD3sXms1Gmsbo%3D
CF Sturino M Labelle (1998) Tetrahedron Lett 39 5891 Occurrence Handle10.1016/S0040-4039(98)01240-4 Occurrence Handle1:CAS:528:DyaK1cXltFeisLw%3D
DC Johnson TS Widlanski (2002) Synthesis 8 809 Occurrence Handle10.1055/s-2002-25757
M Ohno M Yoshioka H Nakai (1984) J Am Chem Soc 106 1133 Occurrence Handle10.1021/ja00316a062
SM Weinreb J Sisko (1991) J Org Chem 56 3210 Occurrence Handle10.1021/jo00010a006
D Ellis (2001) Tetrahedron Asymm 12 1589 Occurrence Handle10.1016/S0957-4166(01)00259-2 Occurrence Handle1:CAS:528:DC%2BD3MXlslerurY%3D
M Thakur A Thakur PV Khadikar CT Supuran (2005) Bioorg Med Chem Lett 15 203 Occurrence Handle10.1016/j.bmcl.2004.10.032 Occurrence Handle1:CAS:528:DC%2BD2cXhtVKrsLnE
V Padmavathi KV Reddy A Padmaja P Venugopalan (2003) J Org Chem 68 1567 Occurrence Handle10.1021/jo020473j Occurrence Handle1:CAS:528:DC%2BD3sXitVCrsQ%3D%3D
AR Massah F Kazemi D Azadi S Farzaneh H Aliyan H Javaherian Naghash AR Momeni (2006) Lett Org Chem 3 235 Occurrence Handle10.2174/157017806775789886 Occurrence Handle1:CAS:528:DC%2BD28XjtVektr4%3D
F Toda K Tanaka (2000) Chem Rev 100 1025 Occurrence Handle10.1021/cr940089p
H Sharghi M Hosseini (2002) Tetrahedron 58 10323 Occurrence Handle10.1016/S0040-4020(02)01417-5 Occurrence Handle1:CAS:528:DC%2BD38XpsVersL0%3D
P Salehi M Dabiri MA Zolfigol M Baghbanzadeh (2005) Tetrahedron Lett 46 7051 Occurrence Handle10.1016/j.tetlet.2005.08.043 Occurrence Handle1:CAS:528:DC%2BD2MXpvVSms7Y%3D
A Shokravi H Sharghi H Vallizadeh MM Heravi (2002) Phosphorus Sulfur Silicon Relat Elem 11 2555 Occurrence Handle10.1080/10426500214560
H Sharghi M Hosseini (2003) Synthesis 9 243 Occurrence Handle10.1055/s-2003-36830
MA Zolfigol D Azarifar B Maleki (2004) Tetrahedron Lett 45 2181 Occurrence Handle10.1016/j.tetlet.2004.01.038 Occurrence Handle1:CAS:528:DC%2BD2cXhtlOqtro%3D
AR Massah M Mosharafian AR Momeni H Aliyan H Javaherian Naghash M Adibnejad (2007) Synth Commun 37 1807 Occurrence Handle10.1080/00397910701316268 Occurrence Handle1:CAS:528:DC%2BD2sXmtlWmtrg%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Massah, A., Azadi, D., Aliyan, H. et al. An Efficient Method for the Synthesis of N-Acylsulfonamides: One-pot Sulfonylation and Acylation of Primary Arylamines under Solvent-Free Conditions. Monatsh Chem 139, 233–240 (2008). https://doi.org/10.1007/s00706-007-0783-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-007-0783-2