Abstract
Objective
Epstein-Barr virus (EBV)-associated gastric cancer (EBVaGC) is a distinct molecular subtype of gastric cancer (GC). At present, the clinical characteristics and prognostic implications of EBV infection and the potential clinical benefits of immune checkpoint blockade in GC remain to be clarified. Hence, this study was designed to analyze the clinical and pathological characteristics of GC patients with varying EBV infection states and compare their overall survival (OS).
Methods
A retrospective study was performed on 1031 consecutive GC patients who underwent gastrectomy at the Affiliated Hospital of Xuzhou Medical University from February 2018 to November 2022. EBV-encoded RNA (EBER) in situ hybridization (ISH) was used for EBV assessment, and immunohistochemical staining was used for evaluation of human epidermal growth factor receptor 2 (HER2), programmed death ligand 1 (PD-L1), and Ki67 expression. EBVaGC was defined as tumors with EBV positivity. In addition, EBV-negative GC (EBVnGC) patients were matched with EBVaGC patients based on seven clinicopathological parameters (age, gender, anatomic subsite, tumor size, Lauren classification, degree of differentiation, and tumor-node-metastasis [TNM] stage). The correlations of clinical features with HER2, PD-L1, and Ki67 expression were evaluated statistically. The survival of patients was assessed through medical records, telephone, or WeChat communication, and prognostic analysis was performed using the logrank test as well as univariable and multivariable regression analysis.
Results
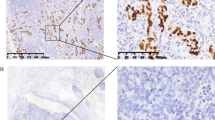
Out of 1031 GC patients tested, 35 (3.4%) were diagnosed with EBVaGC. Notably, the EBVaGC group exhibited a distinct predominance of males and younger patients, significantly higher Ki67 and PD-L1 expression levels, and a lower prevalence of pericancerous nerve invasion than the EBVnGC group (P < 0.01). In the 35 EBVaGC cases, Ki67 expression was negatively correlated with age (P < 0.05), suggesting that a younger onset age was associated with higher Ki67 expression. In addition, PD-L1 expression was correlated with the degree of differentiation, T-stage, and clinical stage of the patient. Furthermore, PD-L1 expression was elevated in tumors with lower differentiation or at later stages (P < 0.05). Using univariate analysis, Ki67, PD-L1, and clinical stage were identified as significant factors influencing the overall survival (OS) of EBVaGC patients (P < 0.05). Moreover, multivariate survival analysis revealed that clinical stage and Ki67 expression were independent risk factors for the OS of the patients (P < 0.05), and the three-year OS rate of EBVaGC patients was 64.2%.
Conclusion
EBV-ISH is a practical and valuable method to identify EBVaGC. Owing to its unique etiological, pathological, and clinical characteristics, patients with EBVaGC might benefit from immune checkpoint blockade therapy.



Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
World Health Organization (WHO). Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019. WHO (2020) Accessed December 11, 2020. whowww.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death
Costache S, Sajin M, Wedden S et al A consolidated working classification of gastric cancer for histopathologists (Review). Biomed Rep. 2023 July 19;19(3):58
Jia X, Guo T, Li Z et al (2021) Clinicopathological and Immunomicroenvironment Characteristics of Epstein-Barr Virus-Associated Gastric Cancer in a Chinese Population. Front Oncol 10:586752
Lieberman PM (2016) Epigenetics and Genetics of Viral Latency. Cell Host Microbe 19(5):619–628
Lu J, Murakami M, Verma SC et al (2011) Epstein-Barr Virus nuclear antigen 1 (EBNA1) confers resistance to apoptosis in EBV-positive B-lymphoma cells through up-regulation of survivin. Virology 410(1):64–75
Frappier L (2012) Contributions of Epstein-Barr nuclear antigen 1 (EBNA1) to cell immortalization and survival. Viruses 4(9):1537–1547
Münz C (2019) Latency and lytic replication in Epstein-Barr virus-associated oncogenesis. Nat Rev Microbiol 17(11):691–700
Shinozaki-Ushiku A, Kunita A, Fukayama M (2015) Update on Epstein-Barr virus and gastric cancer (review). Int J Oncol 46(4):1421–1434. https://doi.org/10.3892/ijo.2015.2856
Farrell PJ Epstein-Barr Virus and Cancer. Annu Rev Pathol 2019 January 24;14:29–53
Burke AP, Yen TS, Shekitka KM, Sobin LH (1990) Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod Pathol 3(3):377–380
Roy S (2021) Physicians’ Dilemma of False-Positive RT-PCR for COVID-19: a Case Report. SN Compr Clin Med 3(1):255–258
AbuSalah MAH, Gan SH, Al-Hatamleh MAI et al (2020) Recent Advances in Diagnostic Approaches for Epstein-Barr Virus. Pathogens 9(3):226
Owliaee I, Khaledian M, Boroujeni AK et al (2023) Engineered small extracellular vesicles as a novel platform to suppress human oncovirus-associated cancers. Infect Agents Cancer 69(18):1750–9378
Tokunaga M, Uemura Y, Tokudome T et al (1993 Oct) Epstein-Barr virus virus-related gastric cancer in Japan: a molecular patho-epidemiological study. Acta Pathol Jpn 43(10):574–581
Chen JN, Ding YG, Feng ZY et al (2010) Association of distinctive Epstein-Barr virus variants with gastric carcinoma in Guangzhou, southern China. J Med Virol 82(4):658–667
Qiu MZ, He CY, Lu SX et al (2020) Prospective observation: Clinical utility of plasma Epstein-Barr virus DNA load in EBV-associated gastric carcinoma patients. Int J Cancer 146(1):272–280
Lee JH, Kim SH, Han SH et al (2009) Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: a meta-analysis. J Gastroenterol Hepatol 24(3):354–365
Camargo MC, Kim WH, Chiaravalli AM et al (2014) Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut 63(2):236–243
Cancer Genome Atlas Research Network (2014) Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513(7517):202–209
Au WY, Pang A, Chan EC et al (2005) Epstein-barr virus-related gastric adenocarcinoma: an early secondary cancer post hemopoietic stem cell transplantation. Gastroenterology 129(6):2058–2063
Hofmann M, Stoss O, Shi D et al (2008) Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 52(7):797–805
Kulangara K, Zhang N, Corigliano E et al (2019) Clinical Utility of the Combined Positive Score for Programmed Death Ligand-1 Expression and the Approval of Pembrolizumab for Treatment of Gastric Cancer. Arch Pathol Lab Med 143(3):330–337
Song HJ, Srivastava A, Lee J et al (2010) Host inflammatory response predicts survival of patients with Epstein-Barr virus-associated gastric carcinoma. Gastroenterology 139(1):84–92e2
Li GH, Zhou ZH, Wang ZX et al (2023) Assessing Epstein-Barr virus in gastric cancer: clinicopathological features and prognostic implications. Infect Agent Cancer 18:11
Atri-Schuller A, Abushukair H, Cavalcante L et al (2022) Tumor Molecular and Microenvironment Characteristics in EBV-Associated Malignancies as Potential Therapeutic Targets: Focus on Gastric Cancer. Curr Issues Mol Biol 44(11):5756–5767
Salnikov MY, Fonseca GJ, Mymryk JS (2023) Differences in the Tumor Microenvironment of EBV-Associated Gastric Cancers Revealed Using Single-Cell Transcriptome Analysis. Cancers (Basel) 15(12):3178
Wong Y, Meehan MT, Burrows SR et al (2022) Estimating the global burden of Epstein-Barr virus-related cancers. J Cancer Res Clin Oncol 148(1):31–46
Tatematsu D, Akao M, Park H et al (2023 March) Relationship between the inclusion/exclusion criteria and sample size in randomized controlled trials for SARS-CoV-2 entry inhibitors. J Theor Biol 21:561:111403
Chen JN, He D, Tang F et al (2012) Epstein-Barr virus-associated gastric carcinoma: a newly defined entity. J Clin Gastroenterol 46(4):262–271
Van Cutsem E, Bang YJ, Feng-Yi F et al (2015) HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 18(3):476–484
Chakravorty S, Afzali B, Kazemian M (2022 October) EBV-associated diseases: Current therapeutics and emerging technologies. Front Immunol 27:13:1059133
Dugan JP, Coleman CB, Haverkos B (2019 March) Opportunities to Target the Life Cycle of Epstein-Barr Virus (EBV) in EBV-Associated Lymphoproliferative Disorders. Front Oncol 15:9:127
Sukawa Y, Yamamoto H, Nosho K et al (2012 December) Alterations in the human epidermal growth factor receptor 2-phosphatidylinositol 3-kinase-v-Akt pathway in gastric cancer. World J Gastroenterol 7(45):6577–6586
Zhang YW, Zhao XX, Tan C et al (2015) Epstein-Barr virus latent membrane protein 2A suppresses the expression of HER2 via a pathway involving TWIST and YB-1 in Epstein-Barr virus-associated gastric carcinomas. Oncotarget 6(1):207–220
Lee HS, Chang MS, Yang HK et al (2004) Epstein-barr virus-positive gastric carcinoma has a distinct protein expression profile in comparison with epstein-barr virus-negative carcinoma. Clin Cancer Res. March 1;10(5):1698–1705
Park JH, Kim EK, Kim YH et al (2016) Epstein-Barr virus positivity, not mismatch repair- deficiency, is a favorable risk factor for lymph node metastasis in submucosa-invasive early gastric cancer. Gastric Cancer 19(4):1041–1051
Osumi H, Kawachi H, Murai K et al (2019) Risk stratification for lymph node metastasis using Epstein-Barr virus status in submucosal invasive (pT1) gastric cancer without lymphovascular invasion: a multicenter observational study. Gastric Cancer 22(6):1176–1182
Zhang Y, Yang Y, Chen Y et al (2022) PD-L1: Biological mechanism, function, and immunotherapy in gastric cancer. Front Immunol 13:1060497
Mishima S, Kawazoe A, Nakamura Y et al (2019) Clinicopathological and molecular features of responders to nivolumab for patients with advanced gastric cancer. J Immunother Cancer 7(1):24
Tsao LC, Force J, Hartman ZC Mechanisms of Therapeutic Antitumor Monoclonal Antibodies. Cancer Res 2021 September 15;81(18):4641–4651
Funding
This work was supported by Xuzhou Medical Key Talent Training Project (XWRCHT20220068); Xuzhou Medical University Hospital Development Fund Innovation Team Project (XYFC2021001), and the surface project (XYFM2021014).
Author information
Authors and Affiliations
Contributions
L.L, A.Y., M.Z., and H.W. conceptualized and designed the study and drafted the initial manuscript. L.M., M.C., W.L., X.Q., and C.G. collected the data and carried out the initial analyses. Z.H. and H.W. critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This study was approved by the Medical Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (XYFY2021-KL063-01).
Additional information
Communicated by Zhongjie Shi.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Ll., Yu, Ay., Zhu, M. et al. Clinicopathological characteristics and prognosis of Epstein-Barr virus–associated gastric cancer. Arch Virol 169, 114 (2024). https://doi.org/10.1007/s00705-024-06033-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00705-024-06033-3