Abstract
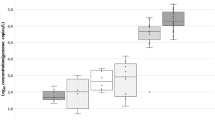
There is increasing evidence that the fecal indicator bacteria that are routinely used for testing water quality are inadequate for ensuring protection of the public health. Pepper mild mottle virus (PMMoV) has recently been suggested as an alternative indicator of human fecal contamination in water; however, in Egypt there are no data available about its occurrence and concentration in aquatic environment. The concentration of PMMoV in the influent and effluent of three wastewater treatment plants was measured using qRT-PCR over a period of one year and compared to that of human adenovirus (HAdV), which is considered an indicator for human fecal contamination. PMMoV was detected in ~ 94% of the influent samples and 78% of the effluent samples, with concentrations ranging from 3.9 × 104 to 3.3 × 108 genome copies/l (GC/l) in the influent and 3.9 × 104 to 1.2 × 107 GC/l in the effluent. Similarly, HAdV was identified in 88% and 78% of the influent and effluent samples, respectively. The HAdV concentration ranged between 1.5 × 104 and 1.5 × 107 GC/l for the influent and 2.6 × 104 and 4.4 × 106 GC/l for the effluent. No significant difference was found between the removal ratio of PMMoV and HAdV. Viral reduction of 0.2-1.9 log10 and 0.2- 2.3 log10 by the treatment process was observed for PMMoV and HAdV, respectively. Both viruses showed no clear seasonality. Our data support the use of PMMoV as a fecal indicator of wastewater contamination and a process indicator for the performance of the treatment process.



Similar content being viewed by others
References
Ahmed W, Hamilton KA, Lobos A, Hughes B, Staley C, Sadowsky MJ, Harwood VJ (2018) Quantitative microbial risk assessment of microbial source tracking markers in recreational water contaminated with fresh untreated and secondary treated sewage. Environ Int 117:243–249
Allard A, Vantarakis A (2017) Adenoviruses. In: Rose JB, Jiménez-Cisneros B (eds) Global water pathogens project, pp 3–30
Bibby K, Peccia J (2013) Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Environ Sci Technol 47:1945–1951
Bibby K, Crank K, Greaves J, Li X, Wu Z, Hamza IA, Stachler E (2019) Metagenomics and the development of viral water quality tools. Clean Water 2:9
Bofill-Mas S, Albinana-Gimenez N, Clemente-Casares P, Hundesa A, Rodriguez-Manzano J, Allard A, Calvo M, Girones R (2006) Quantification and stability of human adenoviruses and polyomavirus JCPyV in wastewater matrices. Appl Environ Microbiol 72:7894–7896
Cantalupo PG, Calgua B, Zhao G, Hundesa A, Wier AD, Katz JP, Grabe M, Hendrix RW, Girones R, Wang D, Pipas JM (2011) Raw sewage harbors diverse viral populations. mBio 2
Carducci A, Morici P, Pizzi F, Battistini R, Rovini E, Verani M (2008) Study of the viral removal efficiency in a urban wastewater treatment plant. Water Sci Technol 58:893–897
Colson P, Richet H, Desnues C, Balique F, Moal V, Grob JJ, Berbis P, Lecoq H, Harle JR, Berland Y, Raoult D (2010) Pepper mild mottle virus, a plant virus associated with specific immune responses, Fever, abdominal pains, and pruritus in humans. PLoS One 5:e10041
Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA (2005) Virus taxonomy. In: Eighth report of the international committee on taxonomy of viruses. Elsevier Academic Press, Amsterdam
Fong TT, Phanikumar MS, Xagoraraki I, Rose JB (2010) Quantitative detection of human adenoviruses in wastewater and combined sewer overflows influencing a Michigan river. Appl Environ Microbiol 76:715–723
Genda Y, Kanda A, Hamada H, Sato K, Ohnishi J, Tsuda S (2007) Two amino acid substitutions in the coat protein of pepper mild mottle virus are responsible for overcoming the L(4) Gene-mediated resistance in Capsicum spp. Phytopathology 97:787–793
Gerba CP, Gramos DM, Nwachuku N (2002) Comparative inactivation of enteroviruses and adenovirus 2 by UV light. Appl Environ Microbiol 68:5167–5169
Gerba CP (2015) Chapter 23 - Indicator Microorganisms. In: Pepper IL, Gerba CP, Gentry TJ (eds) Environmental microbiology, 3rd edn. Academic Press, San Diego, pp 551–564
Gruber JS, Ercumen A, Colford JM Jr (2014) Coliform bacteria as indicators of diarrheal risk in household drinking water: systematic review and meta-analysis. PLoS One 9:e107429
Hamza H, Abd-Elshafy DN, Fayed SA, Bahgat MM, El-Esnawy NA, Abdel-Mobdy E (2017) Detection and characterization of hepatitis A virus circulating in Egypt. Arch Virol 162:1921–1931
Hamza H, Leifels M, Wilhelm M, Hamza IA (2017) Relative abundance of human bocaviruses in urban sewage in Greater Cairo, Egypt. Food Environ Virol 9:304–313
Hamza H, Hamza IA (2018) Oncogenic papillomavirus and polyomavirus in urban sewage in Egypt. Sci Total Environ 610–611:1413–1420
Hamza IA, Jurzik L, Uberla K, Wilhelm M (2011) Evaluation of pepper mild mottle virus, human picobirnavirus and Torque teno virus as indicators of fecal contamination in river water. Water Res 45:1358–1368
Hamza IA, Jurzik L, Überla K, Wilhelm M (2011) Evaluation of pepper mild mottle virus, human picobirnavirus and Torque teno virus as indicators of fecal contamination in river water. Water Res 45:1358–1368
Han TH, Kim SC, Kim ST, Chung CH, Chung JY (2014) Detection of norovirus genogroup IV, klassevirus, and pepper mild mottle virus in sewage samples in South Korea. Adv Virol 159:457–463
Haramoto E, Kitajima M, Katayama H, Ohgaki S (2010) Real-time PCR detection of adenoviruses, polyomaviruses, and torque teno viruses in river water in Japan. Water Res 44:1747–1752
Haramoto E, Kitajima M, Kishida N, Konno Y, Katayama H, Asami M, Akiba M (2013) Occurrence of pepper mild mottle virus in drinking water sources in Japan. Appl Environ Microbiol 79:7413–7418
Harwood VJ, Staley C, Badgley BD, Borges K, Korajkic A (2014) Microbial source tracking markers for detection of fecal contamination in environmental waters: relationships between pathogens and human health outcomes. FEMS Microbiol Rev 38:1–40
Heim A, Ebnet C, Harste G, Pring-Akerblom P (2003) Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J Med Virol 70:228–239
Hewitt J, Greening GE, Leonard M, Lewis GD (2013) Evaluation of human adenovirus and human polyomavirus as indicators of human sewage contamination in the aquatic environment. Water Res 47:6750–6761
Hughes B, Beale DJ, Dennis PG, Cook S, Ahmed W (2017) Cross-comparison of human wastewater-associated molecular markers in relation to fecal indicator bacteria and enteric viruses in recreational beach waters. Appl Environ Microbiol 83:e00028-00017
ICTV (2012) Family - Virgaviridae. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (eds) Virus taxonomy: ninth report of the international committee on taxonomy of viruses. Elsevier, San Diego, pp 1139–1162
Jiang SC (2002) Adenovirus as an index of human viral contamination. US EPA workshop on microbial source tracking: 5 February 2002. Irvine, pp 75–78
Kamel AH, Ali MA, El-Nady HG, Aho S, Pothier P, Belliot G (2010) Evidence of the co-circulation of enteric viruses in sewage and in the population of Greater Cairo. J Appl Microbiol 108:1620–1629
Kitajima M, Iker BC, Pepper IL, Gerba CP (2014) Relative abundance and treatment reduction of viruses during wastewater treatment processes—identification of potential viral indicators. Sci Total Environ 488–489:290–296
Kitajima M, Sassi HP, Torrey JR (2018) Pepper mild mottle virus as a water quality indicator. Clean Water 1:19
Kuroda K, Nakada N, Hanamoto S, Inaba M, Katayama H, Do AT, Nga TT, Oguma K, Hayashi T, Takizawa S (2015) Pepper mild mottle virus as an indicator and a tracer of fecal pollution in water environments: comparative evaluation with wastewater-tracer pharmaceuticals in Hanoi, Vietnam. Sci Total Environ 506–507:287–298
Lee S, Hata A, Yamashita N, Tanaka H (2017) Evaluation of virus reduction by ultrafiltration with coagulation-sedimentation in water reclamation. Food Environ Virol 9:453–463
Nguyen KH, Senay C, Young S, Nayak B, Lobos A, Conrad J, Harwood VJ (2018) Determination of wild animal sources of fecal indicator bacteria by microbial source tracking (MST) influences regulatory decisions. Water Res 144:424–434
Nwachuku N, Gerba CP, Oswald A, Mashadi FD (2005) Comparative inactivation of adenovirus serotypes by UV light disinfection. Appl Environ Microbiol 71:5633–5636
Pina S, Puig M, Lucena F, Jofre J, Girones R (1998) Viral pollution in the environment and in shellfish: human adenovirus detection by PCR as an index of human viruses. Appl Environ Microbiol 64:3376–3382
Rachmadi AT, Kitajima M, Pepper IL, Gerba CP (2016) Enteric and indicator virus removal by surface flow wetlands. Sci Total Environ 542:976–982
Rosario K, Symonds EM, Sinigalliano C, Stewart J, Breitbart M (2009) Pepper mild mottle virus as an indicator of fecal pollution. Appl Environ Microbiol 75:7261–7267
Schmitz BW, Kitajima M, Campillo ME, Gerba CP, Pepper IL (2016) Virus Reduction during advanced bardenpho and conventional wastewater treatment processes. Environ Sci Technol 50:9524–9532
Sinclair RG, Jones EL, Gerba CP (2009) Viruses in recreational water-borne disease outbreaks: a review. J Appl Microbiol 107:1769–1780
Smits SL, Osterhaus AD, Koopmans MP (2016) Newly identified viruses in human gastroenteritis: pathogens or not? Pediatr Infect Dis J 35:104–107
Symonds EM, Verbyla ME, Lukasik JO, Kafle RC, Breitbart M, Mihelcic JR (2014) A case study of enteric virus removal and insights into the associated risk of water reuse for two wastewater treatment pond systems in Bolivia. Water Res 65:257–270
Symonds EM, Young S, Verbyla ME, McQuaig-Ulrich SM, Ross E, Jimenez JA, Harwood VJ, Breitbart M (2017) Microbial source tracking in shellfish harvesting waters in the Gulf of Nicoya, Costa Rica. Water Res 111:177–184
Symonds EM, Nguyen KH, Harwood VJ, Breitbart M (2018) Pepper mild mottle virus: A plant pathogen with a greater purpose in (waste)water treatment development and public health management. Water Res 144:1–12
USEPA (2001) Manual of methods for virology. EPA/600/4-84/013 USEPA, Cincinnati, USA, pp 6–62
Vergara GGRV, Rose JB, Gin KYH (2016) Risk assessment of noroviruses and human adenoviruses in recreational surface waters. Water Res 103:276–282
Villena C, El-Senousy WM, Abad FX, Pinto RM, Bosch A (2003) Group A rotavirus in sewage samples from Barcelona and Cairo: emergence of unusual genotypes. Appl Environ Microbiol 69:3919–3923
Wyn-Jones AP, Carducci A, Cook N, D’Agostino M, Divizia M, Fleischer J, Gantzer C, Gawler A, Girones R, Höller C, de Roda Husman AM, Kay D, Kozyra I, López-Pila J, Muscillo M, José Nascimento MS, Papageorgiou G, Rutjes S, Sellwood J, Szewzyk R, Wyer M (2011) Surveillance of adenoviruses and noroviruses in European recreational waters. Water Res 45:1025–1038
Zhang T, Breitbart M, Lee WH, Run J-Q, Wei CL, Soh SWL, Hibberd ML, Liu ET, Rohwer F, Ruan Y (2005) RNA viral community in human feces: prevalence of plant pathogenic viruses. PLoS Biol 4:e3
Zhang T, Breitbart M, Lee WH, Run JQ, Wei CL, Soh SW, Hibberd ML, Liu ET, Rohwer F, Ruan Y (2006) RNA viral community in human feces: prevalence of plant pathogenic viruses. PLoS Biol 4:e3
Acknowledgements
We thank Kyle Bibby for his valuable comments and suggestions on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Massimo Turina.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hamza, H., Rizk, N.M., Gad, M.A. et al. Pepper mild mottle virus in wastewater in Egypt: a potential indicator of wastewater pollution and the efficiency of the treatment process. Arch Virol 164, 2707–2713 (2019). https://doi.org/10.1007/s00705-019-04383-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-019-04383-x