Abstract
Objective
Neurosurgical indications for the superior eyelid transorbital endoscopic approach (SETOA) are rapidly expanding over the last years. Nevertheless, as any new technique, a detailed knowledge of the anatomy of the surgical target area, the operative corridor, and the specific surgical landmark from this different perspective is required for a safest and successful surgery. Therefore, the aim of this study is to provide, through anatomical dissections, a detailed investigation of the surgical anatomy revealed by SETOA via anterolateral triangle of the middle cranial fossa. We also sought to define the relevant surgical landmarks of this operative corridor.
Methods
Eight embalmed and injected adult cadaveric specimens (16 sides) underwent dissection and exposure of the cavernous sinus and middle cranial fossa via superior eyelid endoscopic transorbital approach. The anterolateral triangle was opened and its content exposed. An extended endoscopic endonasal trans-clival approach (EEEA) with exposure of the cavernous sinus content and skeletonization of the paraclival and parasellar segments of the internal carotid artery (ICA) was also performed, and the anterolateral triangle was exposed. Measurements of the surface area of this triangle from both surgical corridors were calculated in three head specimens using coordinates of its borders under image-guide navigation.
Results
The drilling of the anterolateral triangle via SETOA unfolds a space that can be divided by the course of the vidian nerve into two windows, a wider “supravidian” and a narrower “infravidian,” which reveal different anatomical corridors: a “medial supravidian” and a “lateral supravidian,” divided by the lacerum segment of the ICA, leading to the lower clivus, and to the medial aspect of the Meckel’s cave and terminal part of the horizontal petrous ICA, respectively. The infravidian corridor leads medially into the sphenoid sinus. The arithmetic means of the accessible surface area of the anterolateral triangle were 45.48 ± 3.31 and 42.32 ± 2.17 mm2 through transorbital approach and endonasal approach, respectively.
Conclusion
SETOA can be considered a minimally invasive route complementary to the extended endoscopic endonasal approach to the anteromedial aspect of the Meckel’s cave and the foramen lacerum. The lateral loop of the trigeminal nerve represents a reliable surgical landmark to localize the lacerum segment of the ICA from this corridor. Nevertheless, as any new technique, a learning curve is needed, and the clinical feasibility should be proven.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The superior eyelid endoscopic transorbital approach (SETOA), initially adopted mainly by ophthalmologists for orbital pathologies, is rapidly increasing in popularity among neurosurgeons over the last years, as witnessed by the numerous and heterogeneous anatomical studies and published surgical series concerning intracranial neurosurgical pathologies extending from the anterior skull base to the petrous apex[7, 11, 12, 26, 30, 31, 46]. Indeed, this corridor allows the direct access to the ventral paramedian and lateral aspects of the anterior and middle cranial fossae, in a minimally invasive fashion, with reduced bone destruction, minimal or no brain retraction, no manipulation of neurovascular structures, satisfactory esthetic results, and short hospital stay[3, 8, 31].
Nevertheless, as any new technique, its surgical potential, in terms of exposure and access areas, well-defined anatomical landmarks, pros and cons, pathologies, and patients’ features suitable for this technique, as well as its therapeutic and/or diagnostic role, are being explored. In addition, a learning curve[12], which first of all includes a detailed knowledge of the anatomy of the surgical target area, the operative corridor and the specific surgical landmarks from this different perspective, is required for a safest and successful surgery.
Several technical variants and anatomical corridors revealed by this approach are continuously described[29, 32, 46] with the aim of reaching even more different surgical target areas and planning optimal and tailored-surgical approaches accordingly.
In this scenario, we focused on the anterolateral triangle of the middle fossa as a front-door to the region of foramen lacerum, because of the anatomical and functional inherent advantages which it offers and make it a relatively safe surgical corridor: it comes into endoscopic view immediately after interperiosteal-dural dissection, and it is devoid of vital or highly functional neurovascular structures.
Therefore, this study provides, through anatomical dissections, a detailed investigation of the surgical anatomy revealed by the transorbital endoscopic approach through the anterolateral triangle of the middle cranial fossa, including the foramen lacerum and its adjacent structures. In addition, we sought to define the relevant key surgical landmarks of this operative corridor, also providing comparative surgical nuances with the endoscopic endonasal route to the same target area[5, 21, 22].
We consider that this operative corridor, in isolated or combined manner, may be suitable for several purposes, such as for diagnostic biopsies, gross total, or subtotal resection of lesions involving the medial aspect of the Meckel’s cave with lateral extension into the middle and infratemporal fossae.
Materials and methods
Anatomical dissections were performed at the Laboratory of Skull Base and Micro-neurosurgery in the Weill Cornell Neurosurgical Innovations and Training Center, New York, USA, and EBRIS Laboratory of Neuroanatomy, Salerno, Italy. Eight adult cadaveric specimens (10 sides), embalmed and injected with red and blue latex for the arteriosus and venous blood vessels, respectively, were adequately secured in a rigid three-pin fixation and underwent endoscopic transorbital approach bilaterally, first, and extended endoscopic endonasal transclival approach, then, for a total of 24 surgical procedures (8 EEEA, 16 SETOA). After the initial step under macroscopic visualization, SETOA was performed with a 4 mm in diameter and 18 cm in length, rigid endoscope as optical device, with 0° and 30° rod lenses (Karl Storz, Tuttlingen, Germany), connected to a light source (300 W Xenon, Karl Storz) through a fiberoptic cable and to an HD camera (Endovision Telecam SL; Karl Storz). The entire endonasal transclival approach was a purely endoscopic procedure.
A high-resolution CT scan was performed in 3 head specimens before the dissections and data were uploaded into a neuronavigation system (Brainlab cranial navigation system). Quantitative analysis of the accessible surface area of the anterolateral triangle exposed through each approach was calculated.
Superior eyelid transorbital endoscopic approach (SETOA) to the middle cranial fossa
A SETOA to the middle cranial fossa was performed as previously reported in the pertinent literature[11].
The head specimen was placed in supine and neutral position, 10° flexed and 5° contralaterally rotated.
A skin incision was placed in a superior eyelid wrinkle, and once the orbicularis oculi muscle was cut taking care not to violate the fibers of the levator palpebrae, the dissection was carried in depth up to the superior orbital rim and extended laterally up to the fronto-zygomatic suture. After cutting the periosteum where it became continuous with the periorbita, the dissection continued, with endoscopic assistance, in a subperiosteum/periorbital plane within the orbit until the lateral margin of the inferior and superior orbital fissures. Zygomatico-facial and zygomatico-temporal arteries were identified and cut. At that point, once the zygomatic body and the intraorbital part of the greater sphenoid wing including the sagittal crest[6] were drilled until to expose the temporal pole dura mater, an interperiosteal-dural dissection via meningo-orbital band (MOB)[10] was performed to unlock the lateral wall of the CS up to the gasserian ganglion (GG). After identification and cutting of the middle meningeal artery (MMA), the temporal pole was elevated in extradural fashion, and the peeling of the floor of the middle cranial fossa was completed. Once the midsubtemporal ridge[48] was identified and flattened laterally to the trigeminal nerve lateral loop — the angle described by the lateral margin of V2 and the ventral margin of V3 at the gasserian ganglion[48] — the drilling of the area of the skull base bounded by the lower border of V2, superiorly, the upper border of V3, inferiorly, and by the line which connects foramina rotundum and ovale, defining the anterolateral triangle of the middle cranial fossa[39], and the skeletonization of the vidian canal, completed the dissection procedure (Fig. 1). The exposure of the vidian nerve started by drilling immediately inferiorly to the foramen rotundum in lateral-to-medial and anterior-to-posterior directions to firstly identify the sphenoid sinus and the superior border of the vidian canal and then proceeded posteriorly along the lower and lateral borders of the same canal up to the trigeminal lateral loop and the lacerum foramen. The drilling should occur along the inferior hemicircumference of the vidian canal as the ICA is located along the superior border. Lastly, the skeletonization proceeded between the inferior border of V2 and the superior margin of the vidian canal.
Superior eyelid transorbital endoscopic approach (SETOA) to the middle cranial fossa (right side). a Skin incision on a wrinkle of the upper eyelid and carried in the depth through the orbicularis oculi muscle (OOM) and extended laterally until to expose the fronto-zygomatic suture (FZS); b after the subperiosteal/periorbital dissection was performed up to identify the superior (SOF) and inferior orbital fissures, the corridor between the periorbit (PO) content medially and the greater sphenoid wing (GSW) forming the lateral wall of the orbit, laterally, is exposed; c exposure of the temporal lobe pole dura mater (TLd) and the sagittal crest (SC) after the drilling of the lateral wall of the orbit; d interperiosteal/dural dissection of the cavernous sinus lateral wall and of the middle fossa with identification of the midsubtemporal ridge (MSR) at the base of the anterolateral triangle between the foramina rotundum and ovale; e flattening of the middle fossa floor and exposure of the III and IV cranial nerves, the trigeminal nerve with its ganglion (GG), branches (V1, V2, V3), and lateral loop (LL) inside the periosteal layer; f drilling of the anterolateral triangle with skeletonization of the vidian nerve (vn). (FZS, fronto-zygomatic suture; OOM, orbicularis oculi muscle; GSW, greater sphenoid wing; SOF, superior orbital fissure; PO, periorbit; TLd, temporal lobe dura; SC, sagittal crest; GG, gasserian ganglion; ACP, anterior clinoid process; LL, lateral loop, MSR, mid-subtemporal ridge)
Extended endoscopic endonasal approach (EEEA) to the clivus
An extended endoscopic endonasal transsphenoidal approach was performed as previously reported in the literature[4, 5]. Conversely from the standard endoscopic endonasal transsphenoidal approach, to obtain a wider exposition of the CS, the sphenoidotomy was extended more laterally, and the posterior ethmoidal cells were opened. Furthermore, to expand the operative corridor, the uncinate process was removed, and the bulla ethmoidalis was opened, thus allowing to reach and remove the anterior ethmoid cells. The removal of the posterior ethmoid cells and the anterior wall of the sphenoid sinus allowed to expose the lateral wall of the sphenoid sinus with a direct trajectory, and once it was removed, the CS came into the view. Lastly, the course of the vidian nerve allowed us to reach the lacerum ICA.
Quantitative analysis
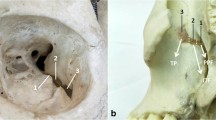
The area of the anterolateral triangle exposed through each approach was calculated using the Brainlab cranial navigation system. For each approach, 3 points — foramen rotundum, foramen ovale and trigeminal nerve lateral loop — corresponding to 3D coordinates obtained by using the stereotactic image-guidance system (Brainlab cranial navigation system), were used as landmark limits of the triangle.
Points registered were expressed as Cartesian coordinates (x, y, z) on the Brainlab workstation. Each point was acquired 3 times, the arithmetic mean for each coordinate was calculated, and a scalene triangle was generated, whose surface area was calculated using the predetermined references at the borders of the triangle which were marked using the navigation device (Fig. 2).
Imagine guidance from neuronavigation system showing measurements of the anterolateral triangle vertices from endonasal (a–c) and transorbital perspectives (d–f). Foramen Rotundum from endonasal (a) and transorbital (d) perspectives; foramen ovale from endonasal (b) and transorbital (e) perspectives; lateral loop of trigeminal nerve from endonasal (c) and transorbital (f) perspectives
Statistical analysis
A Student’s t test was used to compare the mean surface area of the anterolateral triangle of the middle fossa exposed through transorbital and endonasal corridors. A p value ≤ 0.05 was considered statistically significant.
Results
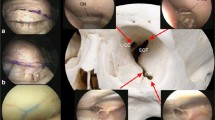
The opening of the anterolateral triangle of the middle cranial fossa through transorbital endoscopic approach allowed us to discover the vidian nerve (vn) and artery in the homonymous canal along their course until the upper part of the anterolateral edge of the foramen lacerum (FL), where the posterior opening of the canal is filled with cartilaginous tissue that blends into the more medially positioned cartilage that fills the foramen lacerum, across the “mandibular strut”[47]. The lacerum segment of the ICA, at its transition zone from the horizontal petrous segment to the posterior ascending cavernous segment, medially to the petrolingual ligament (PL), and the associated carotid sympathetic plexus, could be also exposed (Fig. 3). It was possible to appreciate a space (red dotted lines, Fig. 3a) — quadrangular in shape in 6 out of eight specimens — bounded by the lower border of V2, superiorly, the upper border of V3, posteriorly, by the line crossing the most anterior limit of exposure of the vidian nerve and joining the foramen rotundum and the point where the greater wing joints the body of the sphenoid bone, anteriorly, and the line between the latter point and the foramen ovale posteriorly. The anteroinferior point of this space can be always identified, but it is not a fixed point, and its position depends on the degree of medial retraction of the orbital content; as result, the unfolded space is not always quadrangular in shape.
Exposure of the content of the anterolateral triangle of the middle cranial fossa through SETOA (right side). a Identification of a quadrangular space (red dotted line) and its content (lacerum ICA, vidian nerve, foramen lacerum, lower clivus, medial aspect of Meckel’s cave) limited by the inferior border of V2, superiorly, the superior border of V3, posteriorly, the line crossing the most anterior limit of exposure of the vidian nerve and joining the foramen rotundum and the point where the greater wing joints the body of the sphenoid bone, anteriorly, and the line connecting this last point and the foramen ovale inferiorly; b identification of the supravidian and infravidian windows, divided by the course of the vidian nerve from its distal end of exposure up to its disappearing behind V3, and of the related disclosed corridors: the “medial supravidian” and the “lateral supravidian,” divided by the lacerum segment of ICA, and leading to the lower clivus, and to the medial aspect of the Meckel’s cave and to the distal end of the horizontal segment of the petrous ICA, respectively; c expanded view of the “medial supravidian corridor” after gentle upward displacement of V2; d expanded view of the “lateral supravidian corridor” after gentle lateralization of the gasserian ganglion. (GG, gasserian ganglion; Lac ICA, lacerum internal carotid artery; CLIV, clivus; FL, foramen lacerum; vn, vidian nerve; MC, Meckel’s cave; cICA, cavernous internal carotid artery; pICA, petrous internal carotid artery; LL, lateral loop)
This space allowed us to distinguish two windows divided by the course of the vidian nerve until the point where it blends into the cartilaginous tissue of the FL and which revealed different corridors (Fig. 3):
-
a)
A wider superior window (“supravidian”), which unfolded two corridors divided by the lacerum segment of the ICA: a “medial supravidian corridor,” which leaded in a more lateral-to-medial direction to the lower clivus and which could be expanded after a gentle upward displacement of V2, and a “lateral supravidian corridor” which leaded to the medial aspect of the Meckel’s cave and the terminal portion of the horizontal segment of the petrous ICA (pICA) after gentle lateralization of the gasserian ganglion (GG).
-
b)
A narrower inferior window (“infravidian”), which revealed the lower intracranial part of the foramen lacerum, distally, and the sphenoid sinus proximally and medially.
In all, 8 specimens undergone dissection, and thus in sixteen, transorbital procedures were performed; the lacerum segment of the ICA medially to the distal end of vidian nerve and petrolingual ligament was identified in the depth of the corner between the origin of V2 and V3 from the gasserian ganglion, also known as trigeminal “lateral loop,” and thus at the postero-superior corner of the identified space, along the latero-to-medial trajectory of the transorbital approach.
Quantitative analysis
The arithmetic means of the accessible area of the anterolateral triangle were 45.48 ± 3.31 mm2 and 42.32 ± 2.17 mm2 through transorbital approach and endonasal approach, respectively.
Discussion
The anterolateral triangle of the middle fossa is bounded by the lower border of V2 superiorly, the upper border of V3 inferiorly, and by the line joining the foramina rotundum and ovale anteriorly[39]. Since its first description by Dolenc in 1989[14], while there is unanimous consent on its anatomical limits, there is not agreement on its nomenclature. Furthermore, albeit the size of its drilling is variable according to the natural anatomic variability among patients in physiological conditions, some quantitative anatomic studies, including the present one, clearly described its mean surface area from different surgical perspectives[13, 17, 19, 49] (Table 1): that area in the present study resulted 45.48 ± 3.31 mm2 from transorbital route versus 42.32 ± 2.17 mm2 from endonasal route, with no statistically significant difference between them (p = 0.08).
To the best of our knowledge, the present study is the first one to measure through transorbital corridor the accessible surface area of the anterolateral triangle. These values can variously modify in pathologic conditions, following the primarily or secondary involvement of the parasellar region and displacement of second and third branches of the trigeminal nerve.
When opened from transcranial microsurgical route, mainly through the fronto-temporo-orbito-zygomatic or sub-temporal approaches, the lateral-to-medial trajectory allows the exposure of the vidian canal and its content, namely the vidian nerve and artery and the sphenoid sinus[39].
The opening of the anterolateral triangle through EEEA provides the visualization of the inferomedial temporal dural of the middle cranial fossa[13, 24]. This corridor allows the resection of lesions of the cavernous sinus with anterolateral extension or pituitary adenomas thanks to their softer consistency and so easier to suction out; in addition, this triangle is used to gain access to critical structures, such as anterolateral aspect of the C4 segment of the ICA and the origin of the inferolateral trunk[17].
The drilling of the anterolateral triangle through SETOA ensured the exposure of the same anatomical structures of the transcranial route, but thanks to its anterior-to-posterior and lateral-to-medial trajectories and the capability of the endoscope to bring the eyes of the surgeon close to the surgical field, this approach allowed us to follow the course of the vidian nerve more in the depth, posteriorly, and in a specular direction to it until the lacerum foramen and the related ICA segment and also to explore this area and the adjacent structures. In detail, we identified a “supravidian” window which revealed two different corridors divided by the lacerum segment of the ICA: a “medial supravidian” leading to the lower clivus and which could be expanded by the gentle upward displacement of V2 and a “lateral supravidian” leading to the medial aspect of the Meckel’s cave and the terminal portion of the horizontal petrous ICA and which could be expanded by the gentle lateralization of the gasserian ganglion. The “infravidian” window unfolded the sphenoid sinus and the lower intracranial part of the lacerum foramen, proximally and distally, respectively, along the surgical route.
Several lesions of the skull-base involving the region of the foramen lacerum, both as primary tumors such as chondrosarcomas, or secondary to pathologies affecting the cavernous sinus, Meckel’s cave, petrous apex, clival and petroclival regions, pterygopalatine, and infratemporal fossae, may require the exposure of the foramen lacerum and Meckel’s cave. As this area is hidden underneath the gasserian ganglion, its exposure through the traditional microsurgical approaches, usually classified in anterolateral, posterolateral, and lateral[18, 40, 42, 50], is difficult and requires the full mobilization and/or transection of V3 and gasserian ganglion[50]; indeed, all of these routes share the limit of being unable to access the medial aspect of Meckel’s cave. The access to the anteromedial aspect of the Meckel’s cave requires crossing the anteromedial and anterolateral triangles of the middle fossa[18, 27], but for lesions located posteriorly inside this dural pocket, the transgression of the trigeminal nerve is required.
The medial aspect of the Meckel’s cave, referred to as “quadrangular space” or “front door of Meckel’s cave” by Kassam et al.[22], is better exposed and without the need to the cross cranial nerves and vessels from the anteromedial corridor provided by the endoscopic endonasal approach[9, 16, 22, 34, 38, 43], but often at the expense of the integrity of the vidian nerve [1, 9, 22, 23, 51] and the related ophthalmic complications[36].
Among the several approaches, with related pro and cons, described for accessing Meckel’s cave[43], only two cadaveric laboratory studies have focused on the anatomical and technical implications of the lateral endoscopic orbital route to this area[15, 37], but having as target the antero-lateral and superior aspects of this dural recess. Jeon et al.[20]. report gross total resection in 7 out of 9 patients with Meckel’s cave disease who underwent isolated or combined endoscopic transorbital approach through anteromedial triangle or transorbital extended variant through the anterolateral triangle, with low rate of morbidities. Kong et al. [25] report the use of the anteromedial triangle of the cavernous sinus for type A, C, and D1 tumors according to Samii’s modified classification of trigeminal schwannoma [41] and the use of the anterolateral triangle for type D3 tumors with extension to the infratemporal fossa.
The present anatomical study investigates the transorbital endoscopic corridor to the foramen lacerum and the anteromedial aspect of Meckel’s cave via anterolateral triangle of the middle fossa without violating the integrity of cranial nerves. In our opinion, this pathway could be useful for several purposes: the diagnosis, through biopsy, of lesions of uncertain nature and whose radiologic features can mimic schwannoma, such as sarcoidosis, lymphoma, and inflammatory diseases and then for addressing their management through medical or oncological treatments while avoiding an alternative unnecessary and aggressive surgical approach[2]. Furthermore, this corridor can be adopted for the treatment of tumors like schwannomas of the trigeminal nerve and meningioma involving the medial aspect of the Meckel’s cave and with anterolateral extension, adjecting into the middle fossa, in which the vidian nerve is displaced but not enveloped by the lesion, in which the pattern of growth of the lesion expands the front-door to the transorbital corridor[35], or with downward extension into the infratemporal fossa, and/or when the goal of surgery is a subtotal extent of resection followed by adjuvant treatments. Again, the transorbital route will be very useful in a very small subset of patients which are affected by neurotrophic keratitis due to V1 injury, in which sparing the integrity of the vidian nerve is very important[1]. In contrast, if the vidian nerve is enveloped and not dissociable from the lesion, the sacrifice of the nerve will provide a wider surgical corridor to the foramen lacerum, the lower clivus, and the medial aspect of Meckel’s cave through fusion of the supra and infravidian windows, albeit at the expense of the ophthalmological complications (Fig. 4).
While SETOA to the middle cranial fossa can be considered a complementary route to the preauricular infratemporal and retrosigmoid approaches to access the lateral and posterior aspects of the MC[37], the new corridor explored via anterolateral triangle can be considered a complementary or alternative pathway — in selected patients according to the pathology and patients features — to the endoscopic endonasal transpterygoid approach to the anterior and medial aspects of this dural pocket, avoiding manipulation of the vidian nerve and with shorter working distance[1] (Figs. 5 and 6).
Exposure of the foramen lacerum region through endoscopic transorbital (a, c) and extended endonasal transclival (b, d) corridors before (a–b) and after (c–d) removal of the vidian nerve (left side). The lateral-to-medial trajectory provided by the transorbital approach through the anterolateral triangle represents a complementary surgical route to the medial-to-lateral trajectory provided by the endonasal corridor to the foramen lacerum region. (GG, gasserian ganglion; Lac ICA, lacerum internal carotid artery; CLIV, clivus; FL, foramen lacerum; vn, vidian nerve; LL, lateral loop; PLL, petro-lingual ligament)
Table 2 summarizes the main pros and cons of the microsurgical transcranial anterolateral (fronto-temporo-orbito-zygomatic, FTOZ), extended endoscopic endonasal (EEEA), transpterygoid and endoscopic transorbital (SETOA) approaches in accessing the anteromedial aspect of the Meckel’s cave.
Key surgical landmarks
The knowledge of the exact localization of the ICA, both during the preoperative planning and intraoperatively, is crucial in the management of the skull base lesions close to the clivus and the adjacent areas, regardless of the type of surgical approach adopted.
Although more recently other surgical landmarks have been proposed to identify the lacerum segment of ICA via endoscopic endonasal route, such as the so-called carotid sock [28] and the pterygoclival ligament[44], because of its intracranial localization deep-seated in the ventral paramedian skull base beneath the floor of the sphenoid sinus and its relationship with several anatomical structures that represent the gateway to surgical approaches, i.e., the nasal cavity and maxillary sinus, or target areas, i.e., the cavernous sinus, the petrous apex, the Meckel’s cave, the foramen lacerum, and the petrous carotid — or simply encountered along the surgical corridor, i.e., the pterygopalatine fossa — the vidian nerve is considered the main surgical landmark in various operative procedures, both microsurgical and endoscopic, to the skull base[23, 33, 45].
The transorbital endoscopic approach through the drilling of the anterolateral triangle of the middle cranial fossa allowed us to expose the vidian nerve and follow its course until its disappearing behind V3 and after crossing the anterolateral surface of the lacerum segment of the ICA into the homonymous foramen; therefore, this nerve represents a key surgical landmark to the foramen lacerum and its content, even during transorbital endoscopic surgery.
Nevertheless, sometimes this nerve cannot be used as landmark because it is sacrificed during the operative procedure[1, 9, 22, 23, 51] or because its canal is involved by the lesion.
In this scenario, we identified the “lateral loop” of the trigeminal nerve[48] — the dural bridge between V2 and V3 — as valid alternative or additional constant and reliable landmark to the vidian nerve for identifying the lacerum ICA during SETOA, and we consider the maxillary branch a safe road map. As result, early identification of this landmark and its exposure after interperiosteal-dural dissection of the middle fossa allows the surgical exposure of the foramen lacerum, minimizing the risk of accidental injury to the lacerum ICA.
The foramina rotundum and ovale and the midsubtemporal ridge[48] represent key bony landmarks to identify the anterolateral triangle. Skeletonization of the vidian nerve starts by drilling just inferiorly to the foramen rotundum to identify the most anterior end of exposure of the homonymous nerve; then, it proceeds posteriorly by drilling the lower 180° of the vidian canal, as well-established technique to identify the lacerum segment of the ICA via EEEA[23], up to the foramen lacerum where the vidian nerve disappears behind V3. Lastly, the drilling along the inferior border of V2 up to the trigeminal lateral loop completes the exposure of the vidian nerve. At this point, the lacerum ICA is exposed between the vidian nerve inferiorly and the lateral loop of the trigeminal nerve superiorly.
The transorbital endoscopic route respects the principles of modern skull base minimally invasive techniques: flattening the skull base and using the extradural space to approach the target lesion while reducing brain retraction. Suero Molina et al. [43] in a recent literature review analyze the different surgical approaches to reach Meckel’s cave for tissue sampling of such indeterminate lesions. In this scenario, the endoscopic transorbital route may be considered a further option in the armamentarium of the neurosurgeons dealing with lesions involving not only the lateral but also the anteromedial aspect of Meckel’s cave.
Nevertheless, although SETOA provides several advantages, such as scar hidden within the eyelid crease, no temporalis muscle disruption, a rapid and small craniectomy, straight route to the MC with minimal brain retraction and without violating the cavernous sinus, sparing the vidian nerve avoiding the morbidities related to its injury, a mandatory consideration must be kept in mind when using SETOA: the width of the surgical corridor. This route, while providing wide visualization of the parasellar region, uses a narrow and single if compared to the binostril of the expanded endoscopic endonasal, surgical corridor which imposes limitations on surgical freedom and working angles.
Limitations of this study
Pure anatomical studies have the common limitation related to the cadaveric specimens. The first limit is represented by the small number of specimens used. The properties of cadaveric tissues considerably differ from real anatomy: the consistency of the tissues, variability in size and pneumatization of the sphenoid sinus, the variability in size of the trigeminal ganglion, trajectory of the internal carotid artery and cranial nerves, and bone protuberances of the skull base should be considered. No quantitative data.
Conclusion
The endoscopic transorbital approach via anterolateral triangle of the middle fossa can be considered a minimally invasive route complementary to the extended endoscopic endonasal to the anteromedial aspect of the Meckel’s cave and the foramen lacerum. The angle described by the origin of the maxillary and mandibular divisions of the trigeminal nerve from the gasserian ganglion, also known as trigeminal “lateral loop,” represents a valid surgical landmark to the lacerum segment of the ICA and the course of the maxillary division of the trigeminal nerve a safe road map to it. Nevertheless, as any new operative technique, a learning curve is required and feasibility in a clinical setting must be demonstrated.
Data availability
Data of the current original research are available from the corresponding author on reasonable request.
Abbreviations
- SETOA :
-
Superior eyelid transorbital endoscopic approach
- EEEA :
-
Extended endoscopic endonasal approach
- ICA :
-
Internal carotid artery
- GG :
-
Gasserian ganglion
- MOB :
-
Meningo-orbital band
- MMA :
-
Middle meningeal artery
- CS :
-
Cavernous sinus
- FL :
-
Foramen lacerum
- PL :
-
Petrolingual ligament
- vn :
-
Vidian nerve
- pICA :
-
Petrous internal carotid artery
- FTOZ :
-
Fronto-temporo-orbito-zygomatic
- MC :
-
Meckel’s cave
References
Alves-Belo JT, Mangussi-Gomes J, Truong HQ, Cohen S, Gardner PA, Snyderman CH, Stefko ST, Wang EW, Fernandez-Miranda JC (2019) Lateral transorbital versus endonasal transpterygoid approach to the lateral recess of the sphenoid sinus-a comparative anatomic study. Oper Neurosurg (Hagerstown) 16:600–606. https://doi.org/10.1093/ons/opy211
Bal J, Bruneau M, Berhouma M, Cornelius JF, Cavallo LM, Daniel RT, Froelich S, Jouanneau E, Meling TR, Messerer M, Roche PH, Schroeder HWS, Tatagiba M, Zazpe I, Paraskevopoulos D (2022) Management of non-vestibular schwannomas in adult patients: a systematic review and consensus statement on behalf of the EANS skull base section Part II: Trigeminal and facial nerve schwannomas (CN V, VII). Acta Neurochir (Wien) 164:299–319. https://doi.org/10.1007/s00701-021-05092-8
Balakrishnan K, Moe KS (2011) Applications and outcomes of orbital and transorbital endoscopic surgery. Otolaryngol Head Neck Surg 144:815–820. https://doi.org/10.1177/0194599810397285
Cavallo LM, Cappabianca P, Galzio R, Iaconetta G, de Divitiis E, Tschabitscher M (2005) Endoscopic transnasal approach to the cavernous sinus versus transcranial route: anatomic study. Neurosurgery 56:379–389. https://doi.org/10.1227/01.neu.0000156548.30011.d4. (discussion 379-389)
Cavallo LM, Cappabianca P, Messina A, Esposito F, Stella L, de Divitiis E, Tschabitscher M (2007) The extended endoscopic endonasal approach to the clivus and cranio-vertebral junction: anatomical study. Childs Nerv Syst 23:665–671. https://doi.org/10.1007/s00381-007-0332-7
Corrivetti F, de Notaris M, Di Somma A, Dallan I, Enseñat J, Topczewski T, Solari D, Cavallo LM, Cappabianca P, Prats-Galino A (2022) ″Sagittal crest”: definition, stepwise dissection, and clinical implications from a transorbital perspective. Oper Neurosurg (Hagerstown) 22:e206–e212. https://doi.org/10.1227/ons.0000000000000131
Corvino S, Guizzardi G, Sacco M, Corrivetti F, Bove I, Enseñat J, Colamaria A, Prats-Galino A, Solari D, Cavallo LM, Di Somma A, de Notaris M (2023) The feasibility of three port endonasal, transorbital, and sublabial approach to the petroclival region: neurosurgical audit and multiportal anatomic quantitative investigation. Acta Neurochir (Wien). https://doi.org/10.1007/s00701-023-05498-6
Corvino S, Sacco M, Somma T et al (2023) Functional and clinical outcomes after superior eyelid transorbital endoscopic approach for spheno-orbital meningiomas: illustrative case and literature review. Neurosurg Rev 46:17
Cárdenas Ruiz-Valdepeñas E, Simal Julián JA, Pérez Prat G, Arraez MA, Ambrosiani J, Martin Schrader I, Soto Moreno A, Kaen A (2022) The quadrangular space, endonasal access to the Meckel cave: technical considerations and clinical series. World Neurosurg 163:e124–e136. https://doi.org/10.1016/j.wneu.2022.03.077
Dallan I, Di Somma A, Prats-Galino A, Solari D, Alobid I, Turri-Zanoni M, Fiacchini G, Castelnuovo P, Catapano G, de Notaris M (2017) Endoscopic transorbital route to the cavernous sinus through the meningo-orbital band: a descriptive anatomical study. J Neurosurg 127:622–629. https://doi.org/10.3171/2016.8.JNS16465
Di Somma A, Andaluz N, Cavallo LM, Topczewski TE, Frio F, Gerardi RM, Pineda J, Solari D, Enseñat J, Prats-Galino A, Cappabianca P (2018) Endoscopic transorbital route to the petrous apex: a feasibility anatomic study. Acta Neurochir (Wien) 160:707–720. https://doi.org/10.1007/s00701-017-3448-x
Di Somma A, Kong DS, de Notaris M, Moe KS, Sánchez España JC, Schwartz TH, Enseñat J (2022) Endoscopic transorbital surgery levels of difficulty. J Neurosurg 1–4. doi:https://doi.org/10.3171/2022.3.JNS212699
Dolci RL, Upadhyay S, Ditzel Filho LF, Fiore ME, Buohliqah L, Lazarini PR, Prevedello DM, Carrau RL (2016) Endoscopic endonasal study of the cavernous sinus and quadrangular space: anatomic relationships. Head Neck 38(Suppl 1):E1680-1687. https://doi.org/10.1002/hed.24301
Dolenc V (1989) Anatomy and Surgery of the Cavernous Sinus. 1 edn. Springer-Verlag/Wien 1989. doi:https://doi.org/10.1007/978-3-7091-6942-1
Ferrari M, Schreiber A, Mattavelli D, Belotti F, Rampinelli V, Lancini D, Doglietto F, Fontanella MM, Tschabitscher M, Rodella LF, Nicolai P (2016) The inferolateral transorbital endoscopic approach: a preclinical anatomic study. World Neurosurg 90:403–413. https://doi.org/10.1016/j.wneu.2016.03.017
Fortes FS, Sennes LU, Carrau RL, Brito R, Ribas GC, Yasuda A, Rodrigues AJ, Snyderman CH, Kassam AB (2008) Endoscopic anatomy of the pterygopalatine fossa and the transpterygoid approach: development of a surgical instruction model. Laryngoscope 118:44–49. https://doi.org/10.1097/MLG.0b013e318155a492
Granger A, Bricoune O, Rajnauth T, Kimball D, Kimball H, Tubbs RS, Loukas M (2018) Anterolateral triangle: a cadaveric study with neurosurgical significance. Cureus 10:e2185. https://doi.org/10.7759/cureus.2185
Inoue T, Rhoton AL, Theele D, Barry ME (1990) Surgical approaches to the cavernous sinus: a microsurgical study. Neurosurgery 26:903–932. https://doi.org/10.1097/00006123-199006000-00001
Isolan GR, Krayenbühl N, de Oliveira E, Al-Mefty O (2007) Microsurgical anatomy of the cavernous sinus: measurements of the triangles in and around it. Skull Base 17:357–367. https://doi.org/10.1055/s-2007-985194
Jeon C, Hong CK, Woo KI, Hong SD, Nam DH, Lee JI, Choi JW, Seol HJ, Kong DS (2018) Endoscopic transorbital surgery for Meckel's cave and middle cranial fossa tumors: surgical technique and early results. J Neurosurg 1–10. doi:https://doi.org/10.3171/2018.6.JNS181099
Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL (2005) Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus 19:E4
Kassam AB, Prevedello DM, Carrau RL, Snyderman CH, Gardner P, Osawa S, Seker A, Rhoton AL (2009) The front door to meckel's cave: an anteromedial corridor via expanded endoscopic endonasal approach- technical considerations and clinical series. Neurosurgery 64:ons71–82; discussion ons82–73. doi:https://doi.org/10.1227/01.NEU.0000335162.36862.54
Kassam AB, Vescan AD, Carrau RL, Prevedello DM, Gardner P, Mintz AH, Snyderman CH, Rhoton AL (2008) Expanded endonasal approach: vidian canal as a landmark to the petrous internal carotid artery. J Neurosurg 108:177–183. https://doi.org/10.3171/JNS/2008/108/01/0177
Komatsu F, Oda S, Shimoda M, Imai M, Shigematsu H, Komatsu M, Tschabitscher M, Matsumae M (2014) Endoscopic endonasal approach to the middle cranial fossa through the cavernous sinus triangles: anatomical considerations. Neurol Med Chir (Tokyo) 54:1004–1008. https://doi.org/10.2176/nmc.oa.2014-0092
Kong D, Kim, Y. H., Lee, W., Kim, Y., & Hong, C. (2022) Indications and outcomes of endoscopic transorbital surgery for trigeminal schwannoma based on tumor classification: a multicenter study with 50 cases. J Neurosurg (published online ahead of print 2022). https://thejns.org/view/journals/j-neurosurg/aop/article-10.3171-2022.9.JNS22779/article-10.3171-2022.9.JNS22779.xml
Kong DS, Kim YH, Hong CK (2020) Optimal indications and limitations of endoscopic transorbital superior eyelid surgery for spheno-orbital meningiomas. J Neurosurg 134:1472–1479. https://doi.org/10.3171/2020.3.JNS20297
Krisht AF (2005) Transcavernous approach to diseases of the anterior upper third of the posterior fossa. Neurosurg Focus 19:E2
Labib MA, Prevedello DM, Carrau R, Kerr EE, Naudy C, Abou Al-Shaar H, Corsten M, Kassam A (2014) A road map to the internal carotid artery in expanded endoscopic endonasal approaches to the ventral cranial base. Neurosurgery 10(Suppl 3):448–471. https://doi.org/10.1227/NEU.0000000000000362. (discussion 471)
Lim J, Sung KS, Kim W, Yoo J, Jung IH, Choi S, Lim SH, Roh TH, Hong CK, Moon JH (2021) Extended endoscopic transorbital approach with superior-lateral orbital rim osteotomy: cadaveric feasibility study and clinical implications (SevEN-007). J Neurosurg:1–14. doi:https://doi.org/10.3171/2021.7.JNS21996
López CB, Di Somma A, Cepeda S, Arrese I, Sarabia R, Agustín JH, Topczewski TE, Enseñat J, Prats-Galino A (2021) Extradural anterior clinoidectomy through endoscopic transorbital approach: laboratory investigation for surgical perspective. Acta Neurochir (Wien) 163:2177–2188. https://doi.org/10.1007/s00701-021-04896-y
Moe KS, Bergeron CM, Ellenbogen RG (2010) Transorbital neuroendoscopic surgery. Neurosurgery 67:ons16-28. https://doi.org/10.1227/01.NEU.0000373431.08464.43
Noiphithak R, Yanez-Siller JC, Revuelta Barbero JM, Otto BA, Carrau RL, Prevedello DM (2019) Comparative analysis between lateral orbital rim preservation and osteotomy for transorbital endoscopic approaches to the cavernous sinus: an anatomic study. Oper Neurosurg (Hagerstown) 16:86–93. https://doi.org/10.1093/ons/opy054
Osawa S, Rhoton AL, Seker A, Shimizu S, Fujii K, Kassam AB (2009) Microsurgical and endoscopic anatomy of the vidian canal. Neurosurgery 64:385–411. https://doi.org/10.1227/01.NEU.0000338945.54863.D9. (discussion 411-382)
Palejwala SK, Zhao F, Lanker KC, Sivakumar W, Takasumi Y, Griffiths CF, Barkhoudarian G, Kelly DF (2018) Imaging-ambiguous lesions of Meckel’s cave-utility of endoscopic endonasal transpterygoid biopsy. World Neurosurg 118:e346–e355. https://doi.org/10.1016/j.wneu.2018.06.190
Park HH, Hong SD, Kim YH, Hong CK, Woo KI, Yun IS, Kong DS (2020) Endoscopic transorbital and endonasal approach for trigeminal schwannomas: a retrospective multicenter analysis (KOSEN-005). J Neurosurg 133:467–476. https://doi.org/10.3171/2019.3.JNS19492
Prevedello DM, Pinheiro-Neto CD, Fernandez-Miranda JC, Carrau RL, Snyderman CH, Gardner PA, Kassam AB (2010) Vidian nerve transposition for endoscopic endonasal middle fossa approaches. Neurosurgery 67:478–484. https://doi.org/10.1227/NEU.0b013e3181faaa70
Priddy BH, Nunes CF, Beer-Furlan A, Carrau R, Dallan I, Prevedello DM (2017) A side door to Meckel’s cave: anatomic feasibility study for the lateral transorbital approach. Oper Neurosurg (Hagerstown) 13:614–621. https://doi.org/10.1093/ons/opx042
Raza SM, Donaldson AM, Mehta A, Tsiouris AJ, Anand VK, Schwartz TH (2014) Surgical management of trigeminal schwannomas: defining the role for endoscopic endonasal approaches. Neurosurg Focus 37:E17. https://doi.org/10.3171/2014.7.FOCUS14341
Rhoton AL (2002) The cavernous sinus, the cavernous venous plexus, and the carotid collar. Neurosurgery 51:S375-410
Samii M, Carvalho GA, Tatagiba M, Matthies C (1997) Surgical management of meningiomas originating in Meckel’s cave. Neurosurgery 41:767–774. https://doi.org/10.1097/00006123-199710000-00003. (discussion 774-765)
Samii M, Migliori MM, Tatagiba M, Babu R (1995) Surgical treatment of trigeminal schwannomas. J Neurosurg 82:711–718. https://doi.org/10.3171/jns.1995.82.5.0711
Samii M, Tatagiba M, Carvalho GA (2000) Retrosigmoid intradural suprameatal approach to Meckel’s cave and the middle fossa: surgical technique and outcome. J Neurosurg 92:235–241. https://doi.org/10.3171/jns.2000.92.2.0235
Suero Molina E, Revuelta Barbero JM, Ewelt C, Stummer W, Carrau RL, Prevedello DM (2021) Access to Meckel’s cave for biopsies of indeterminate lesions: a systematic review. Neurosurg Rev 44:249–259. https://doi.org/10.1007/s10143-020-01247-w
Tayebi Meybodi A, Little AS, Vigo V, Benet A, Kakaizada S, Lawton MT (2018) The pterygoclival ligament: a novel landmark for localization of the internal carotid artery during the endoscopic endonasal approach. J Neurosurg:1–11. doi:https://doi.org/10.3171/2017.12.JNS172435
Tubbs RS, Salter EG (2006) Vidius Vidius (Guido Guidi): 1509–1569. Neurosurgery 59:201–203. https://doi.org/10.1227/01.NEU.0000219238.52858.47. (discussion 201-203)
Vural A, Carobbio ALC, Ferrari M, Rampinelli V, Schreiber A, Mattavelli D, Doglietto F, Buffoli B, Rodella LF, Taboni S, Tomasoni M, Gualtieri T, Deganello A, Hirtler L, Nicolai P (2021) Transorbital endoscopic approaches to the skull base: a systematic literature review and anatomical description. Neurosurg Rev. https://doi.org/10.1007/s10143-020-01470-5
Wang WH, Lieber S, Mathias RN, Sun X, Gardner PA, Snyderman CH, Wang EW, Fernandez-Miranda JC (2018) The foramen lacerum: surgical anatomy and relevance for endoscopic endonasal approaches. J Neurosurg:1–12. doi:https://doi.org/10.3171/2018.6.JNS181117
Wanibuchi M, Murakami G, Yamashita T, Minamida Y, Fukushima T, Friedman AH, Fujimiya M, Houkin K (2011) Midsubtemporal ridge as a predictor of the lateral loop formed by the maxillary nerve and mandibular nerve: a cadaveric morphological study. Neurosurgery 69:95–98. https://doi.org/10.1227/NEU.0b013e31821247f5. (discussion ons98)
Watanabe A, Nagaseki Y, Ohkubo S, Ohhashi Y, Horikoshi T, Nishigaya K, Nukui H (2003) Anatomical variations of the ten triangles around the cavernous sinus. Clin Anat 16:9–14. https://doi.org/10.1002/ca.10072
Yasuda A, Campero A, Martins C, Rhoton AL, de Oliveira E, Ribas GC (2005) Microsurgical anatomy and approaches to the cavernous sinus. Neurosurgery 56:4–27. https://doi.org/10.1227/01.neu.0000144208.42171.02. (discussion 24-27)
Zanation AM, Snyderman CH, Carrau RL, Gardner PA, Prevedello DM, Kassam AB (2009) Endoscopic endonasal surgery for petrous apex lesions. Laryngoscope 119:19–25. https://doi.org/10.1002/lary.20027
Acknowledgements
Thanks to the Laboratory of Skull Base and Micro-neurosurgery of the Weill Cornell Neurosurgical Innovations and Training Center, New York, USA.
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Sergio Corvino: conception, anatomical dissection, data collection, data review, drafting manuscript. Daniele Armocida, Giovanni Pennisi, Benedetta Burattini, Andres Villareal Mondragon: anatomical dissections. Felice Esposito, Luigi Maria Cavallo, Matteo de Notaris: study supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Any portion of the paper has not been previously elsewhere presented nor published.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Corvino, S., Armocida, D., Offi, M. et al. The anterolateral triangle as window on the foramen lacerum from transorbital corridor: anatomical study and technical nuances. Acta Neurochir 165, 2407–2419 (2023). https://doi.org/10.1007/s00701-023-05704-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05704-5