Abstract
Background
Diffuse hemispheric glioma, H3 G34-mutant, is a novel paediatric tumour type in the fifth edition of the WHO classification of CNS tumours associated with an invariably poor outcome. We present a comprehensive clinical, imaging and pathological review of this entity.
Methods
Patients with confirmed H3 G34R-mutant high-grade glioma were included in a single-centre retrospective cohort study and examined for clinical, radiological and histo-molecular data.
Results
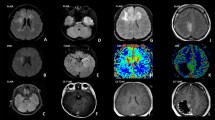
Twelve patients were enrolled in the study — 7 males/5 females; the mean age was 17.5 years (10–57 years). Most patients presented with signs of raised intracranial pressure (8/12). The frontal lobe (60%) was the prevalent location, with a mixed cystic-nodular appearance (10/12) and presence of vascular flow voids coursing through/being encased by the mass (8/12), and all tumours showed cortical invasion. Nine patients had subtotal resection limited by functional margins, two patients underwent supra-total resection, and one patient had biopsy only. 5-ALA was administered to 6 patients, all of whom showed positive fluorescence. Histologically, the tumours showed a marked heterogeneity and aggressive spread along pre-existing brain structures and leptomeninges. In addition to the diagnostic H3 G34R/V mutation, pathogenic variants in TP53 and ATRX genes were found in most cases. Potential targetable mutations in PDGFRA and PIK3CA genes were detected in five cases. The MGMT promoter was highly methylated in half of the samples. Methylation profiling was a useful diagnostic tool and highlighted recurrent structural chromosome abnormalities, such as PDGFRA amplification, CDKN2A/B deletion, PTEN loss and various copy number changes in the cyclin D-CDK4/Rb pathway. Radiochemotherapy was the most common adjuvant treatment (9/12), and the average survival was 19.3 months.
Conclusions
H3 G34R-mutant hemispheric glioma is a distinct entity with characteristic imaging and pathological features. Genomic landscaping of individual tumours can offer an opportunity to adapt individual therapies and improve patient management.




Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Baig Mirza A, Christodoulides I, Lavrador JP et al (2021) 5-Aminolevulinic acid-guided resection improves the overall survival of patients with glioblastoma-a comparative cohort study of 343 patients. Neurooncol Adv 3(1):vdab047
Chen CCL, Deshmukh S, Jessa S et al (2020) Histone H3.3G34-mutant interneuron progenitors co-opt PDGFRA for gliomagenesis. Cell 183(6):1617-1633.e22
Hegi ME, Diserens A-C, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352(10):997–1003
Hu W, Duan H, Zhong S, Zeng J, Mou Y (2022) High frequency of PDGFRA and MUC family gene mutations in diffuse hemispheric glioma, H3 G34-mutant: a glimmer of hope? J Transl Med 20(1):64
Kallappagoudar S, Yadav RK, Lowe BR, Partridge JF (2015) Histone H3 mutations–a special role for H3.3 in tumorigenesis? Chromosoma 124(2):177–189
Korshunov A, Capper D, Reuss D et al (2016) Histologically distinct neuroepithelial tumors with histone 3 G34 mutation are molecularly similar and comprise a single nosologic entity. Acta Neuropathol 131(1):137–146
Kurokawa R, Baba A, Kurokawa M, Pinarbasi ES, Makise N, Ota Y, Kim J, Srinivasan A, Moritani T (2022) Neuroimaging features of diffuse hemispheric glioma, H3 G34-mutant: a case series and systematic review. J Neuroimaging 32(1):17–27
Lee J, Solomon DA, Tihan T (2017) The role of histone modifications and telomere alterations in the pathogenesis of diffuse gliomas in adults and children. J Neurooncol 132(1):1–11
Lim KY, Won JK, Park C-K, Kim S-K, Choi SH, Kim T, Yun H, Park S-H (2021) H3 G34-mutant high-grade glioma. Brain Tumor Pathol 38(1):4–13
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131(6):803–820
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 23(8):1231–1251
Lowe BR, Maxham LA, Hamey JJ, Wilkins MR, Partridge JF (2019) Histone H3 mutations: an updated view of their role in chromatin deregulation and cancer. Cancers (Basel). https://doi.org/10.3390/cancers11050660
Lucas C-HG, Mueller S, Reddy A et al (2021) Diffuse hemispheric glioma, H3 G34-mutant: genomic landscape of a new tumor entity and prospects for targeted therapy. Neuro Oncol 23(11):1974–1976
Mackay A, Burford A, Carvalho D et al (2017) Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 32(4):520-537.e5
Mazurowski MA, Desjardins A, Malof JM (2013) Imaging descriptors improve the predictive power of survival models for glioblastoma patients. Neuro Oncol 15(10):1389–1394
Morris M, Driscoll M, Henson JW, Cobbs C, Jiang L, Gocke CD, Chen L, Rodriguez FJ (2020) Low-grade gemistocytic morphology in H3 G34R-mutant gliomas and concurrent K27M mutation: clinicopathologic findings. J Neuropathol Exp Neurol 79(10):1038–1043
Picart T, Barritault M, Poncet D et al (2021) Characteristics of diffuse hemispheric gliomas, H3 G34-mutant in adults. Neurooncol Adv 3(1):vdab061
Puntonet J, Dangouloff-Ros V, Saffroy R, Pagès M, Andreiuolo F, Grill J, Puget S, Boddaert N, Varlet P (2018) Historadiological correlations in high-grade glioma with the histone 3.3 G34R mutation. J Neuroradiol 45(5):316–322
Roussel MF, Robinson GW (2013) Role of MYC in medulloblastoma. Cold Spring Harb Perspect Med. https://doi.org/10.1101/cshperspect.a014308
Schwartzentruber J, Korshunov A, Liu X-Y et al (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482(7384):226–231
Solomon DA, Wood MD, Tihan T, Bollen AW, Gupta N, Phillips JJJ, Perry A (2016) Diffuse midline gliomas with histone H3–K27M mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol 26(5):569–580
Sturm D, Witt H, Hovestadt V et al (2012) Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22(4):425–437
Vettermann FJ, Felsberg J, Reifenberger G et al (2018) Characterization of diffuse gliomas with histone H3–G34 mutation by MRI and dynamic 18F-FET PET. Clin Nucl Med 43(12):895–898
Wang Y, Qian T, You G et al (2015) Localizing seizure-susceptible brain regions associated with low-grade gliomas using voxel-based lesion-symptom mapping. Neuro Oncol 17(2):282–288
Wang L, Shao L, Li H et al (2022) Histone H3.3 G34-mutant diffuse gliomas in adults. Am J Surg Pathol 46(2):249–257
Wu G, Broniscer A, McEachron TA et al (2012) Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet 44(3):251–253
Yoshimoto K, Hatae R, Sangatsuda Y et al (2017) Prevalence and clinicopathological features of H3.3 G34-mutant high-grade gliomas: a retrospective study of 411 consecutive glioma cases in a single institution. Brain Tumor Pathol 34(3):103–112
Zhang R-Q, Shi Z, Chen H et al (2016) Biomarker-based prognostic stratification of young adult glioblastoma. Oncotarget 7(4):5030–5041
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper has not been previously presented or published
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lavrador, J.P., Reisz, Z., Sibtain, N. et al. H3 G34-mutant high-grade gliomas: integrated clinical, imaging and pathological characterisation of a single-centre case series. Acta Neurochir 165, 1615–1633 (2023). https://doi.org/10.1007/s00701-023-05545-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05545-2