Abstract
Background
Deep brain stimulation (DBS) is a well-established treatment for Parkinson’s disease (PD). While the success of DBS is dependent on careful patient selection and accurate lead placement, programming parameters play a pivotal role in tailoring therapy on the individual level. Various algorithms have been developed to streamline the initial programming process, but the relationship between pre-operative patient characteristics and post-operative device settings is unclear. In this study, we investigated how PD severity correlates with DBS settings.
Methods
We conducted a retrospective review of PD patients who underwent DBS of the subthalamic nucleus at one US tertiary care center between 2014 and 2018. Pre-operative patient characteristics and post-operative programming data at various intervals were collected. Disease severity was measured using the Unified Parkinson’s Disease Rating Scale score (UPDRS) as well as levodopa equivalent dose (LED). Correlation analyses were conducted looking for associations between pre-operative disease severity and post-operative programming parameters.
Results
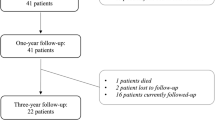
Fifty-six patients were analyzed. There was no correlation between disease severity and any of the corresponding programming parameters. Pre-operative UPDRS scores on medication were similar to post-operative scores with DBS. Settings of amplitude, frequency, and pulse width increased significantly from 1 to 6 months post-operatively. Stimulation volume, inferred by the distance between contacts used, also increased significantly over time.
Conclusions
Interestingly, we found that patients with more advanced disease responded to electrical stimulation similarly to patients with less advanced disease. These data provide foundational knowledge of DBS programming parameters used in a single cohort of PD patients over time.





Similar content being viewed by others
Abbreviations
- DBS:
-
Deep brain stimulation
- PD:
-
Parkinson’s disease
- UPDRS:
-
Unified Parkinson’s Disease Rating Scale
- LED:
-
Levodopa equivalent dose
- STN:
-
Subthalamic nucleus
References
Accolla E, Caputo E, Cogiamanian F, Tamma F, Mrakic-Sposta S, Marceglia S, Egidi M, Rampini P, Locatelli M, Priori A (2007) Gender differences in patients with Parkinson’s disease treated with subthalamic deep brain stimulation. Mov Disord 22(8):1150–1156
Annic A, Moreau C, Salleron J, Devos D, Delval A, Dujardin K, Touzet G, Blond S, Destée A, Defebvre L (2014) Predictive factors for improvement of gait by low-frequency stimulation in Parkinson’s disease. J Parkinsons Dis 4(3):413–420
Aubignat M, Lefranc M, Tir M, Krystkowiak P (2020) Deep brain stimulation programming in Parkinson’s disease: introduction of current issues and perspectives. Rev Neurol (Paris) 176(10):770–779
Dayal V, Limousin P, Foltynie T (2017) Subthalamic nucleus deep brain stimulation in Parkinson’s disease: the effect of varying stimulation parameters. J Parkinsons Dis 7(2):235–245
Di GI, Kalliolia E, Georgiev D, Peters AL, Voyce DC, Akram H, Foltynie T, Limousin P, Day BL (2019) Chronic subthalamic nucleus stimulation in Parkinson’s disease: optimal frequency for gait depends on stimulation site and axial symptoms. Front Neurol. https://doi.org/10.3389/FNEUR.2019.00029
Gorodetsky C, Fasano A (2021) Basic tips: how do I start programming deep brain stimulation in Parkinson disease patients? Mov Disord Clin Pract 8(4):639–644
Heldman DA, Pulliam CL, Mendoza EU, Gartner M, Giuffrida JP, Montgomery EB Jr, Espay AJ, Revilla FJ (2016) Computer-guided deep brain stimulation programming for Parkinson’s disease. Neuromodulation 19(2):127
Jiang L, Chen W, Guo Q et al (2021) Eight-year follow-up outcome of subthalamic deep brain stimulation for Parkinson’s disease: maintenance of therapeutic efficacy with a relatively low levodopa dosage and stimulation intensity. CNS Neurosci Ther 27(11):1366–1373
Khazen O, Dimarzio M, Platanitis K et al (2021) Sex-specific effects of subthalamic nucleus stimulation on pain in Parkinson’s disease. J Neurosurg 135(2):629–636
Koeglsperger T, Palleis C, Hell F, Mehrkens JH, Bötzel K (2019) Deep brain stimulation programming for movement disorders: current concepts and evidence-based strategies. Front Neurol. https://doi.org/10.3389/FNEUR.2019.00410
Montuno MA, Kohner AB, Foote KD, Okun MS (2013) An algorithm for management of deep brain stimulation battery replacements: devising a web-based battery estimator and clinical symptom approach. Neuromodulation Technol Neural Interface 16(2):147–153
Okun MS, Tagliati M, Pourfar M, Fernandez HH, Rodriguez RL, Alterman RL, Foote KD (2005) Management of referred deep brain stimulation failures: a retrospective analysis from 2 movement disorders centers. Arch Neurol 62(8):1250–1255
Ondo WG, Meilak C, Vuong KD (2007) Predictors of battery life for the Activa® Soletra 7426 Neurostimulator. Park Relat Disord 13(4):240–242
Paek SH, Kim HJ, Yoon JY et al (2011) Fusion image-based programming after subthalamic nucleus deep brain stimulation. World Neurosurg 75(3–4):517–524
Palleis C, Gehmeyr M, Mehrkens JH, Bötzel KKT (2020) Establishment of a visual analog scale for DBS programming (VISUAL-STIM Trial). Front Neurol. https://doi.org/10.3389/FNEUR.2020.561323
Park YS, Kim HY, Chang WS, Lee PH, Sohn YH, Chang JW (2010) A comparison of LEDD and motor scores following STN-DBS treatment in patient with young onset vs. late onset Parkinson’s disease. Neuromodulation Technol Neural Interface 13(4):255–260
Peralta M, Haegelen C, Jannin P, Baxter JSH (2021) PassFlow: a multimodal workflow for predicting deep brain stimulation outcomes. Int J Comput Assist Radiol Surg 16(8):1361–1370
Picillo M, Lozano AM, Kou N, Puppi Munhoz R, Fasano A (2016) Programming deep brain stimulation for Parkinson’s disease: the Toronto Western Hospital algorithms. Brain Stimul 9(3):425–437
Pourfar MH, Mogilner AY, Farris S, Giroux M, Gillego M, Zhao Y, Blum D, Bokil H, Pierre MC (2015) Model-based deep brain stimulation programming for Parkinson’s disease: the GUIDE pilot study. Stereotact Funct Neurosurg 93(4):231–239
Santaniello S, Gale JT, Sarma SV (2018) Systems approaches to optimizing deep brain stimulation therapies in Parkinson’s disease. Wiley Interdiscip Rev Syst Biol Med 10(5):e1421
Schor JS, Nelson AB (2019) Multiple stimulation parameters influence efficacy of deep brain stimulation in parkinsonian mice. J Clin Invest 129(9):3833
Shamir RR, Dolber T, Noecker AM, Walter BL, McIntyre CC (2015) Machine learning approach to optimizing combined stimulation and medication therapies for Parkinson’s disease. Brain Stimul 8(6):1025
Shukla AW, Zeilman P, Fernandez H, Bajwa JA, Mehanna R (2017) DBS programming: an evolving approach for patients with Parkinson’s disease. Parkinsons Dis. https://doi.org/10.1155/2017/8492619
Xu C, Zhuang P, Hallett M, Zhang Y, Li J, Li Y (2018) Parkinson’s disease motor subtypes show different responses to long-term subthalamic nucleus stimulation. Front Hum Neurosci. https://doi.org/10.3389/FNHUM.2018.00365
Zhang D, Lin H, Liu J, Liu Z, Yan J, Cai X (2019) Electrode reconstruction assists postoperative contact selection in deep brain stimulation. World Neurosurg 125:e442–e447
Zibetti M, Merola A, Rizzi L et al (2011) Beyond nine years of continuous subthalamic nucleus deep brain stimulation in Parkinson’s disease. Mov Disord 26(13):2327–2334
Author information
Authors and Affiliations
Contributions
Drs. Girgis and Shahlaie designed and conducted the study. Dr. Zhang, Ms. Sardo, Ms. Sperry, and Mr. Ovruchesky collected data. Drs. Saez and Far performed statistical analyses. Dr. Far prepared the manuscript with important intellectual input from the other authors. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Ethics approval was obtained from the Institutional Review Board (IRB #1440605).
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Comments
The authors of this study analyzed the programming settings in a large single-center cohort of Parkinson’s disease patients treated with subthalamic nucleus (STN) deep brain stimulation (DBS). Despite common stereotype that more severe symptoms require higher stimulation settings, the authors discovered that the correlation between disease severity and stimulation settings is lacking; although the settings were increased during initial 6 months after the implantation, they subsequently plateaued for the duration of follow-up.
Based on review of the presented data, one may wonder if the explanation is more complex than it appears and the real reason for this lack of correlation is the progressive atrophy of the stimulated targets (that may require less electricity to be suppressed), or gradual normalization of stimulated networks that develops over time (and therefore maintains stable stimulation response despite disease progression), or some other mechanism that is yet to be elucidated.
Nevertheless, I applaud the authors for their thorough analysis and want to encourage them and others to maintain critical thinking in processing the wealth of patients’ data in prospective fashion. It is, indeed, conceivable that, similar to recent developments in spinal cord stimulation arena, we will come up to a conclusion that “less is more” and that settings need to be reduced rather than raised when the symptoms change with disease progression.
Konstantin Slavin.
Chicago, USA.
This article is part of the Topical Collection on Functional Neurosurgery—Movement disorders
Rights and permissions
About this article
Cite this article
Far, R., Saez, I., Sardo, A. et al. Subthalamic nucleus deep brain stimulation programming settings do not correlate with Parkinson’s disease severity. Acta Neurochir 164, 2271–2278 (2022). https://doi.org/10.1007/s00701-022-05279-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-022-05279-7