Abstract
Background
Glioblastoma multiforme is the most frequent malignant brain tumor in adults being marked with a very poor prognosis. Therapy concept implies concomitant radio-chemotherapy and facultative implantation of carmustine-eluted wafer. Current literature suggests microRNA 26a expression in glioblastoma to interact with alkylating chemotherapy. Subsequently, the aim of this study was to investigate the correlation of miRNA-26a expression and carmustine wafer implantation and its potential usefulness as a predictive marker for therapy response.
Methods
In total, 229 patients with glioblastoma multiforme were included into the final analysis. Of them, 80 cases were recruited from the Saarland University Medical Center for a retrospective matched-pair analysis stratified after therapy regime: One group (carmustine wafer group; n=40) received concomitant radio-chemotherapy with carmustine wafer implantation. The other group (control group; n=40) only received concomitant radio-chemotherapy. The results were confirmed by comparing them with an independent dataset of 149 patients from the TCGA database. All tumor specimens were evaluated for miRNA-26a expression, MGMT promoter methylation, and IDH1 R132H mutation status, and the results were correlated with the clinical data.
Results
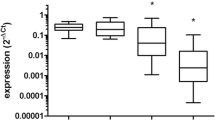
Twenty-three patients in the carmustine wafer group showed low expression of miRNA-26a, while 17 patients showed a high expression. In the control group, 28 patients showed low expression, while 12 patients showed a high expression. The patients with high miRNA-26a expression in the carmustine wafer group were characterized by a significantly longer overall (hazard ratio [HR] 2.750 [95% CI 1.352–5.593]; p=0.004) and progression-free survival (HR 3.091 [95% CI 1.436–6.657]; p=0.003) than patients with low miRNA-26a expression. The 17 patients in the carmustine wafer group with high miRNA-26a expression showed a significantly longer progression-free survival (p=0.013) and overall survival (p=0.007) compared with the control group. There were no such correlations identified within the control group. TCGA datasets supported these findings.
Conclusions
MiRNA-26a expression turned out to be a promising predictor of therapy response and clinical outcome in glioblastoma patients treated with carmustine wafer implantation. For evaluation of the role of miRNA-26a in a combined therapy setting, further studies are needed in order to translate general findings to the patient’s individual situation.




Similar content being viewed by others
Availability of data
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- FC:
-
Fold change
- GBM:
-
Glioblastoma multiforme
- GTR:
-
Gross total resection
- HR:
-
Hazard ratio
- KPS:
-
Karnofsky Performance Score
- MGMT:
-
O6-Methylguanine-DNA methyltransferase
- miRNA:
-
MicroRNA
- MRI:
-
Magnetic resonance imaging
- MS-PCR:
-
Methylation-specific polymerase chain reaction
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- qRT-PCR:
-
Quantitative reverse-transcription polymerase chain reaction
- STR:
-
Subtotal resection
- TCGA:
-
The Cancer Genome Atlas
- TMZ:
-
Temozolomide
References
Abd-El-Barr MM, Chiocca EA (2014) How much is enough? The question of extent of resection in glioblastoma multiforme. World Neurosurg 82(1–2):e109–e110
Ahir BK, Ozer H, Engelhard HH, Lakka SS (2017) MicroRNAs in glioblastoma pathogenesis and therapy: a comprehensive review. Crit Rev Oncol Hematol 120:22–33
Bäcklund LM, Nilsson BR, Goike HM, Schmidt EE, Liu L, Ichimura K, Collins VP (2003) Short postoperative survival for glioblastoma patients with a dysfunctional Rb1 pathway in combination with no wild-type PTEN. Clin Cancer Res Off J Am Assoc Cancer Res 9(11):4151–4158
Bäcklund LM, Nilsson BR, Liu L, Ichimura K, Collins VP (2005) Mutations in Rb1 pathway-related genes are associated with poor prognosis in anaplastic astrocytomas. Br J Cancer 93(1):124–130
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Busato F, Dejeux E, El Abdalaoui H, Gut IG, Tost J (2018) Quantitative DNA methylation analysis at single-nucleotide resolution by pyrosequencing®. Methods Mol Biol Clifton NJ 1708:427–445
Cancer Genome Atlas Research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455(7216):1061–1068
Capper D, Weissert S, Balss J et al (2010) Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol Zurich Switz 20(1):245–254
Chaichana KL, Cabrera-Aldana EE, Jusue-Torres I, Wijesekera O, Olivi A, Rahman M, Quinones-Hinojosa A (2014) When gross total resection of a glioblastoma is possible, how much resection should be achieved? World Neurosurg 82(1–2):e257–e265
Chen C-Z (2005) MicroRNAs as oncogenes and tumor suppressors. N Engl J Med 353(17):1768–1771
Colaprico A, Silva TC, Olsen C et al (2016) TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res 44(8):e71
Curran WJ, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, Chang CH, Rotman M, Asbell SO, Krisch RE (1993) Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst 85(9):704–710
De Bonis P, Anile C, Pompucci A, Fiorentino A, Balducci M, Chiesa S, Maira G, Mangiola A (2012) Safety and efficacy of Gliadel wafers for newly diagnosed and recurrent glioblastoma. Acta Neurochir 154(8):1371–1378
Felsberg J, Rapp M, Loeser S, Fimmers R, Stummer W, Goeppert M, Steiger H-J, Friedensdorf B, Reifenberger G, Sabel MC (2009) Prognostic significance of molecular markers and extent of resection in primary glioblastoma patients. Clin Cancer Res Off J Am Assoc Cancer Res 15(21):6683–6693
Ge X, Pan M-H, Wang L, Li W, Jiang C, He J, Abouzid K, Liu L-Z, Shi Z, Jiang B-H (2018) Hypoxia-mediated mitochondria apoptosis inhibition induces temozolomide treatment resistance through miR-26a/Bad/Bax axis. Cell Death Dis 9(11):1128
Gil J, Peters G (2006) Regulation of the INK4b-ARF-INK4a tumour suppressor locus: all for one or one for all. Nat Rev Mol Cell Biol 7(9):667–677
Goldhoff P, Clarke J, Smirnov I, Berger MS, Prados MD, James CD, Perry A, Phillips JJ (2012) Clinical stratification of glioblastoma based on alterations in retinoblastoma tumor suppressor protein (RB1) and association with the proneural subtype. J Neuropathol Exp Neurol 71(1):83–89
Grabowski MM, Recinos PF, Nowacki AS, Schroeder JL, Angelov L, Barnett GH, Vogelbaum MA (2014) Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg 121(5):1115–1123
Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, Fisher J, NABTT CNS Consortium (2010) Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res Off J Am Assoc Cancer Res 16(8):2443–2449
Halani SH, Babu R, Adamson DC (2017) Management of glioblastoma multiforme in the elderly: a review of the literature. World Neurosurg. https://doi.org/10.1016/j.wneu.2017.04.153
Hartmann C, Meyer J, Balss J et al (2009) Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol (Berl) 118(4):469–474
Hegi ME, Diserens A-C, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352(10):997–1003
Hegi ME, Liu L, Herman JG, Stupp R, Wick W, Weller M, Mehta MP, Gilbert MR (2008) Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol Off J Am Soc Clin Oncol 26(25):4189–4199
Henriksen M, Johnsen KB, Olesen P, Pilgaard L, Duroux M (2014) MicroRNA expression signatures and their correlation with clinicopathological features in glioblastoma multiforme. NeuroMolecular Med 16(3):565–577
Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB (1996) Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A 93(18):9821–9826
Holt DE, Bernard ME, Quan K, Clump DA, Engh JA, Burton SA, Heron DE (2016) Salvage stereotactic radiosurgery for recurrent glioblastoma multiforme with prior radiation therapy. J Cancer Res Ther 12(4):1243–1248
Huse JT, Brennan C, Hambardzumyan D et al (2009) The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev 23(11):1327–1337
Limentani SA, Asher A, Heafner M, Kim JW, Fraser R (2005) A phase I trial of surgery, Gliadel wafer implantation, and immediate postoperative carboplatin in combination with radiation therapy for primary anaplastic astrocytoma or glioblastoma multiforme. J Neuro-Oncol 72(3):241–244
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods San Diego Calif 25(4):402–408
López-Urrutia E, Coronel-Hernández J, García-Castillo V et al (2017) MiR-26a downregulates retinoblastoma in colorectal cancer. Tumour Biol J Int Soc Oncodevelopmental Biol Med 39(4):1010428317695945
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol (Berl) 131(6):803–820
Malzkorn B, Wolter M, Liesenberg F, Grzendowski M, Stühler K, Meyer HE, Reifenberger G (2010) Identification and functional characterization of microRNAs involved in the malignant progression of gliomas. Brain Pathol Zurich Switz 20(3):539–550
Nakamura M, Yonekawa Y, Kleihues P, Ohgaki H (2001) Promoter hypermethylation of the RB1 gene in glioblastomas. Lab Investig J Tech Methods Pathol 81(1):77–82
Nikolova T, Roos WP, Krämer OH, Strik HM, Kaina B (2017) Chloroethylating nitrosoureas in cancer therapy: DNA damage, repair and cell death signaling. Biochim Biophys Acta Rev Cancer 1868(1):29–39
Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS (2013) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro-Oncol 15(Suppl 2):ii1–i56
Pallud J, Audureau E, Noel G et al (2015) Long-term results of carmustine wafer implantation for newly diagnosed glioblastomas: a controlled propensity-matched analysis of a French multicenter cohort. Neuro-Oncol 17(12):1609–1619
Parker NR, Correia N, Crossley B, Buckland ME, Howell VM, Wheeler HR (2013) Correlation of microRNA 132 up-regulation with an unfavorable clinical outcome in patients with primary glioblastoma multiforme treated with radiotherapy plus concomitant and adjuvant temozolomide chemotherapy. Transl Oncol 6(6):742–748
Sabel M, Giese A (2008) Safety profile of carmustine wafers in malignant glioma: a review of controlled trials and a decade of clinical experience. Curr Med Res Opin 24(11):3239–3257
Sawaya R (1999) Extent of resection in malignant gliomas: a critical summary. J Neuro-Oncol 42(3):303–305
Simpson DJ, Hibberts NA, McNicol AM, Clayton RN, Farrell WE (2000) Loss of pRb expression in pituitary adenomas is associated with methylation of the RB1 CpG island. Cancer Res 60(5):1211–1216
Sippl C, Ketter R, Bohr L, Kim YJ, List M, Oertel J, Urbschat S (2018) MiRNA-181d expression significantly affects treatment responses to carmustine wafer implantation. Neurosurgery. https://doi.org/10.1093/neuros/nyy214
Sippl C, Urbschat S, Kim YJ, Senger S, Oertel J, Ketter R (2018) Promoter methylation of RB1, P15, P16, and MGMT and their impact on the clinical course of pilocytic astrocytomas. Oncol Lett 15(2):1600–1606
Stewart LA (2002) Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet Lond Engl 359(9311):1011–1018
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Takeuchi K, Hoshino K (1977) Statistical analysis of factors affecting survival after glioblastoma multiforme. Acta Neurochir 37(1–2):57–73
Weber EL, Goebel EA (2005) Cerebral edema associated with Gliadel wafers: two case studies. Neuro-Oncol 7(1):84–89
Wemmert S, Bettscheider M, Alt S, Ketter R, Kammers K, Feiden W, Steudel W-I, Rahnenführer J, Urbschat S (2009) p15 promoter methylation—a novel prognostic marker in glioblastoma patients. Int J Oncol 34(6):1743–1748
Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jääskeläinen J, Ram Z (2003) A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-Oncol 5(2):79–88
Xu W, Yang H, Liu Y et al (2011) Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 19(1):17–30
Yoshioka M, Matsutani T, Hara A, Hirono S, Hiwasa T, Takiguchi M, Iwadate Y (2018) Real-time methylation-specific PCR for the evaluation of methylation status of MGMT gene in glioblastoma. Oncotarget 9(45):27728–27735
Yu N, Yang Y, Li X, Zhang M, Huang J, Wang X, Long X (2016) MiR-26a inhibits proliferation and migration of HaCaT keratinocytes through regulating PTEN expression. Gene 594(1):117–124
Zhang Y-F, Zhang A-R, Zhang B-C, Rao Z-G, Gao J-F, Lv M-H, Wu Y-Y, Wang S-M, Wang R-Q, Fang D-C (2013) MiR-26a regulates cell cycle and anoikis of human esophageal adenocarcinoma cells through Rb1-E2F1 signaling pathway. Mol Biol Rep 40(2):1711–1720
Acknowledgements
The authors wish to thank Lisa Senger for her editorial support and Sigrid Welsch for their technical assistance in miRNA and methylation analysis.
Funding
This study was financed by Archimedes Pharma (L204150209).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in this study were in accordance with the ethical standards of the 1964 Helsinki declaration. This article does not contain any studies with animals performed by any of the authors.
Ethics approval and consent to participate
This study was approved by the local German ethical board (Ethikkommission der Ärztekammer des Saarlandes, Saarbrücken, Germany).
Consent for publication
Written informed consent was obtained from all patients (General Medical Council of the State of Saarland, NO 93/16).
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Tumor - Glioma
Rights and permissions
About this article
Cite this article
Sippl, C., Ketter, R., Braun, L. et al. miRNA-26a expression influences the therapy response to carmustine wafer implantation in patients with glioblastoma multiforme. Acta Neurochir 161, 2299–2309 (2019). https://doi.org/10.1007/s00701-019-04051-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-019-04051-8