Abstract
A case with cerebral venous air embolism (CVAE) after neurosurgery and treated with hyperbaric oxygen therapy (HBOT) is presented. This is a rare and potentially fatal complication that neurosurgeons should be aware of. A 52-year-old male was diagnosed with an intracerebral hematoma. An emergency evacuation of the hematoma was performed with a craniotomy and the postoperative CT scan showed a complete evacuation of the hematoma, but it also revealed a CVAE. The patient was immediately referred to HBOT and received three sessions within 48 h. The CT scan after the first HBOT showed no CVAE, venous thrombosis, or new hematoma.
Similar content being viewed by others
Introduction
Air embolism is a well-known risk of several medical procedures. A rare and feared subtype of air embolism is the cerebral venous air embolism (CVAE) [13]. It can occur during brain surgery when the integrity of the venous system is broken and the head is elevated above the heart [14]. Other possible causes include during central venous line insertion, use, or removal, or during intravenous drug and fluid infusion [7]. Hyperbaric oxygen therapy (HBOT) is the recommended management for cerebral arterial air embolism [17], but the efficacy of this treatment is not well documented in CVAE [1]. To our knowledge, HBOT for the management of CVAE has only been described twice previously [2, 9].
We present a case with CVAE in the superior sagittal sinus, confluence of sinuses, and transverse sinus after brain surgery which was treated with HBOT.
Case report
Clinical history
A 52-year-old man with a known history of untreated hypertension was admitted to his local hospital due to acute onset of dysarthria and dysphagia and paralysis of his right arm during skiing. On the initial clinical examination, his blood pressure was 188/100 mmHg, Glasgow Coma Scale (GCS) 15, and National Institutes of Health Stroke Scale (NIHSS) 19. The CT scan revealed an intracerebral hematoma (ICH) in the left hemisphere, measuring 44 ml involving the basal ganglia and the internal capsule with a mild midline shift (Fig. 1a). CT angiography revealed no vascular malformation. At this time, there was no indication for acute evacuation of the hematoma and the patient was admitted for observation in the local hospital (with no neurosurgical service). Fourteen hours later, the patient’s GCS dropped to 9 (M6, V2, O1). A new CT scan showed progression of the original hematoma 44 to 52 ml and a midline shift of 6 mm (Fig. 1b). The patient was transferred to our institution by helicopter for neurosurgical intervention. On arrival at OUS-Ullevål, the GCS was 9, blood pressure was 140/95 mmHg, and he had a right-sided hemi-paralysis. The patient was intubated and sedated and a central venous catheter (CVC) was put in the right subclavian vein before transfer to the operating room for craniotomy and hematoma evacuation.
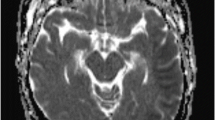
a The initial CT scan (coronal) showing ICH in the left hemisphere measuring 44 ml with slight mass effect. b CT scan (coronal) 14 h later showing expansion of the original ICH measuring 4 × 4 × 4 cm (52 ml) and a midline shift of 6 mm. c CT scan (coronal) postoperative status after evacuation of hematoma. d, e Postoperative CT scan 11 h after surgery showing air in the superior sagittal sinus, confluence of sinuses, and transverse sinuses. f CT cerebral venography (sagittal) after the first HBOT showing no air in the superior sagittal sinus
Surgical procedure
Surgery was performed under general anesthesia using total intravenous anesthesia (TIVA). He was placed in a supine position with the upper body elevated 20° and the head fixed in a Mayfield clamp. A left-sided frontal craniotomy was made. The hematoma was located with intraoperative ultrasound and was evacuated according to standard microsurgical procedure (Fig. 1c). He also got implanted an intracranial pressure (ICP)-monitoring device. The surgery was uneventful with no visible or recognized bleeding from large veins or bone sinuses.
Postoperative course
The operation was completed at 8:30 PM, and he was kept intubated and sedated overnight. The next day at 7:30 am, a routine postoperative CT scan showed a CVAE in the superior sagittal sinus, confluence of sinuses, and transverse sinus (Fig. 1d, e). He was still intubated and sedated and on a ventilator; hence, a neurological examination was not possible. He was hemodynamically stable and received 40% oxygen, before receiving 100% oxygen in anticipation of HBOT. It took 7 h from the discovery of CVAE until the patient was moved to the hyperbaric chamber, located in the same building as our intensive care unit (ICU), for HBOT.
Hyperbaric oxygen therapy
A hyperbaric chamber with ICU capabilities was used for the management. He received a total of three sessions of HBOT. The treatment schedules are adopted from the standard US Navy recompression treatment tables 6 and 9 [6]. The first session consisted of a total of 4 h 50 min and included pressurization to 2.8 atmospheres absolute (ATA), equivalent to 18 m below sea level (bsl) for 1 h 15 min hours followed by 3 h 5 min at 1.9 ATA (9 m bsl). CT venography immediately after the first HBOT showed satisfactory conditions without signs of thrombosis, air emboli, or rebleeding (Fig. 1f). Due to volatile intracranial pressures necessitating continuous neurointensive care, including sedation, a clinical neurological assessment was not possible at the time. With the aim to prevent and ameliorate inflammation, edema, and ischemia-reperfusion injury in the tissues affected by the air embolism, two additional HBOT sessions of 90 min of pressurization at 2.4 ATA (14 m bsl) were administered over the following 36 h. During all HBOT sessions, the patient was ventilated with 100% oxygen, except for short breaks at lower FiO2 as mandated in the treatment tables. An anesthesiologist accompanied the patient in the chamber during all the sessions. Pressurization was tolerated well by the patient and he remained stable with respect to ventilation and circulation throughout. In order to prevent lung atelectasis resulting from prolonged ventilation with 100% oxygen, a PEEP of 10 cm H2O as well as alveolar recruitment maneuvers was administered during and after the hyperbaric sessions. The patient required extended care in the neurosurgical intensive care unit, chiefly due to elevated ICP. He was treated for pneumonia and a tracheostomy tube was placed before he was weaned off ventilator support 13 days after surgery. Upon discharge to the local hospital 17 days after admission, his clinical status was as follows: right-sided hemi-paralysis, right-sided central facial paralysis, neglect, and dysarthria.
Status at last follow-up
After 4 months of rehabilitation, the patient was mobilizing well and was making progress in becoming independent in normal daily activities. He has no dysarthria, dysphagia, or cognitive deficits, except mild fatigue, and he returned to his academic profession in a 40% position.
Discussion
Incidence of CVAE
The incidence of CVAE after intracranial surgery is largely unknown [15]. We routinely do postoperative CT or MR scans on all our patients after intracranial surgery, from 6 to 48 h after surgery, and we very rarely see CVAE.
Causes of CVAE
Air in the cerebral venous system could enter through injured intracerebral vessels or venous channels in the skull bone during neurosurgery, or through an open peripheral or central venous catheter. We did not recognize any injury to bridging veins/sinuses or open venous channels in the bone during the operation. Procedures such as central venous catheter (CVC) insertion, use, or removal also constitute a special risk [7]. The patient should be placed briefly in a Trendelenburg position during CVC placement and removal to counteract this effect [7]. Nedelmann et al. also describe insufficiency in jugular valves as a cause of CVAE [12]. Unfortunately, our case did not receive jugular sonography. The exact cause of CVAE in our patient is not known.
Symptoms of CVAE
CVAE will cause symptoms depending on the size, speed of formation, and location. The outcome ranges from no symptoms to focal neurological deficits and even death. Although CVAE is rare, it should be suspected in patients having acute focal neurological deficits after craniotomy and/or insertion/use/removal of a CVC [5].
Pathophysiology of CVAE
An air or gas embolism may cause mechanical occlusion of the vessel lumen as well as irritation and damage to the vascular endothelium [10]. Although the mechanical occlusion in CVAE does not cause immediate arterial ischemia, the interaction with the endothelium may trigger neutrophil aggregation via β2 integrin adhesion, worsening venous stasis, potentially leading to infarction. An inflammatory cascade mediated by ischemia-reperfusion mechanisms may further exacerbate the injury [3, 11].
Treatment
Initial treatment for any vascular air embolism includes immediate administration of oxygen, aiming for an effective inspired fraction of 100% [8]. The resulting increased partial pressure of oxygen will accelerate nitrogen resorption and decrease bubble size. Placement of the patient in a Trendelenburg position should theoretically move the bubble out of the cerebral veins and towards the pulmonary vascular filter [13]. The application of this maneuver must however be balanced against the potential deleterious effect of increased intracranial pressure.
Fundamentally, HBOT exerts two effects on the body. First, compression by increased atmospheric pressure will mechanically affect any gas bubbles. Their volume is reduced in inverse proportion to the pressure, as dictated by Boyle’s law. Increasing the atmospheric pressure from 1.0 to 2.8 ATA will for example reduce the volume of an air bubble 2.8 times. In the case of a spherical bubble, its diameter will be reduced by a little more than a third and its surface area by half. However, intravascular bubbles of clinical relevance will typically present with a cylindrical shape. Such a sausage-shaped air bubble will by the same pressure increase have its length reduced 2.8 times. In addition, the increased gas pressure of the emboli will help diffuse the nitrogen and oxygen into the tissues and venous blood for removal via the lungs during the HBOT treatment, with gradually reduced pressure according to protocol.
The second effect is a dramatically increased partial pressure of oxygen. At 2.8 ATA, breathing 100% oxygen results in an arterial pO2 of 1800 mmHg. The immense partial pressure gradient between oxygen and nitrogen promotes elimination of nitrogen from the bubble and rapidly reduces its volume [8]. Oxygen delivery is improved in the ischemic penumbra zone and intracranial pressure is reduced due to cerebral artery constriction. Hyperbaric oxygen also has potent anti-inflammatory properties including inhibition of leukocyte-endothelial cell adhesion in injured tissues by HBOT-induced downregulation of β2 integrin cell adhesion molecules [3]. Furthermore, HBOT has been shown in animal and human models to impair proinflammatory cytokine production by monocytes/macrophages and in several models to alter hypoxia-inducible factor-1 (HIF-1) production, improving ischemic tolerance [4, 16].
For arterial gas embolism, repeated HBOT treatment is recommended until no further clinical improvement is observed. No evidence-based recommendations exist for CVAE but we believe a similar approach is reasonable. A neurological examination of our patient was not possible in the first days after surgery and the number of treatments was judged to be appropriate based on our clinical experience treating gas emboli.
Risk of HBOT treatment
There are a few absolute contraindications for HBOT treatment, but previous bleomycin treatment, certain other drugs, and untreated pneumothorax are absolute contraindications.
HBOT is generally safe and well tolerated, and most of the side effects are mild and reversible [4].
Conclusion
We present a potential fatal but rare complication after brain surgery and show that HBOT can be used to treat CVAE even with many hours delay. Very few cases of this have been reported and this is a complication all neurosurgeons must be aware of and its potential outcome.
The message
-
1.
Cerebral venous air embolism (CVAE) after intracranial surgery is a rare.
-
2.
CVAE is a potentially fatal complication.
-
3.
The recommended treatment for CVAE is hyperbaric oxygen therapy (HBOT).
References
Bothma P, Schlimp C (2014) II. Retrograde cerebral venous gas embolism: are we missing too many cases? Br J Anaesth 112:401–404
Bothma PA, Brodbeck AE, Smith BA (2012) Cerebral venous air embolism treated with hyperbaric oxygen: a case report. Diving Hyperb Med 42:101–103
Buras JA, Reenstra WR (2007) Endothelial-neutrophil interactions during ischemia and reperfusion injury: basic mechanisms of hyperbaric oxygen. Neurol Res 29:127–131
Camporesi EM, Bosco G (2014) Mechanisms of action of hyperbaric oxygen therapy. Undersea Hyperb Med 41:247–252
Chang C-C, Chao Y-K, Wu Y-M, Wong H-F, Wong Y-C, Toh C-H (2014) Retrograde cerebral venous air embolism: a case report and review of literature. J Radiol Sci 39:101–104
Diving A (1980) US navy diving manual. Navy Department, Washington, DC 1:7
Heckmann JG, Lang CJ, Kindler K, Huk W, Erbguth FJ, Neundörfer B (2000) Neurologic manifestations of cerebral air embolism as a complication of central venous catheterization. Crit Care Med 28:1621–1625
Jain KK, SpringerLink (2017) Textbook of hyperbaric medicine. 6th ed. 2016. Edn. Springer, Cham
Lai D, Jovin TG, Jadhav AP (2013) Cortical vein air emboli with gyriform infarcts. JAMA Neurol 70:939–940
Moon RE (2014) Hyperbaric oxygen treatment for air or gas embolism. Undersea Hyperb Med 41:159–166
Muth CM, Shank ES (2000) Gas embolism. N Engl J Med 342:476–482
Nedelmann M, Pittermann P, Gast KK, Mueller-Forell W, Dieterich M (2007) Involvement of jugular valve insufficiency in cerebral venous air embolism. J Neuroimaging 17:258–260
Schlimp CJ, Bothma PA, Brodbeck AE (2014) Cerebral venous air embolism: what is it and do we know how to deal with it properly? JAMA Neurol 71:243–243
Schmidts MB, Lederer W, Rieger M, Loimer T, Schlimp CJ (2005) The potential of venous air embolism ascending retrograde to the brain. J Forensic Sci 50:1–4
Shaikh N, Ummunisa F (2009) Acute management of vascular air embolism. J Emerg Trauma Shock 2:180–185
Thom SR (2011) Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg 127(Suppl 1):131S–141S
Trytko B, Bennett M (2008) Arterial gas embolism: a review of cases at Prince of Wales Hospital, Sydney, 1996 to 2006. Anaesth Intensive Care 36:60–64
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. We have obtained informed written consent from the patient for publication of this case report.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Comments
Here, we have a useful and informative clinical case report where fast thinking and fast action most likely prevented a disastrous outcome. These authors did not identify clinical findings of CVAE in this single case, but the CT scan appearance leaves no doubt. A rapid protocol for HBO was instituted, and ultimately, the patient made a reasonable recovery. I have never seen such a case myself and, therefore, have never used HBO for this purpose, but I learned from this report and think that all cranial and trauma surgeons should keep this in mind when evaluating postoperative CT findings. It is a most instructive case.
Christopher Miranda Loftus
Philadelphia, USA
Rights and permissions
About this article
Cite this article
Lundborg, M., Helseth, E., Josefsen, R. et al. Hyperbaric oxygen therapy of air embolus in the cerebral venous sinuses after intracranial surgery: a case report. Acta Neurochir 160, 1401–1405 (2018). https://doi.org/10.1007/s00701-018-3537-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-018-3537-5