Abstract
Background
Experimental CNS trauma results in post-traumatic inflammation for which microglia and macrophages are vital. Experimental brain contusion entails iNOS synthesis and formation of free radicals, NO and peroxynitrite. Shock wave trauma can be used as a model of high-energy trauma in cell culture. It is known that shock wave trauma causes sub-lytic injury and inflammatory activation in endothelial cells. Mechanical disruption of red blood cells can induce iNOS synthesis in experimental systems. However, it is not known whether trauma can induce activation and iNOS synthesis in inflammatory cell lines with microglial or macrophage lineage. We studied the response and activation in two macrophage cell lines and the consequence for iNOS and NO formation after shock wave trauma.
Methods
Two macrophage cell lines from rat (NR8383) and mouse (RAW264.7) were exposed to shock wave trauma by the Flyer Plate method. The cellular response was investigated by Affymetrix gene arrays. Cell survival and morphological activation was monitored for 24 h in a Cell-IQ live cell imaging system. iNOS induction and NO synthesis were analyzed by Western blot, in cell Western IR-immunofluorescence, and Griess nitrite assay.
Results
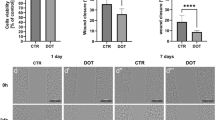
Morphological signs of activation were detected in both macrophage cell lines. The activation of RAW264.7 was statistically significant (p < 0.05), but activation of NR8383 did not pass the threshold of statistical significance alpha (p > 0.05). The growth rate of idle cells was unaffected and growth arrest was not seen. Trauma did not result in iNOS synthesis or NO induction. Gene array analyses showed high enrichment for inflammatory response, G-protein coupled signaling, detection of stimulus and chemotaxis. Shock wave trauma combined with low LPS stimulation instead led to high enrichment in apoptosis, IL-8 signaling, mitosis and DNA-related activities. LPS/IFN-ɣ stimulation caused iNOS and NO induction and morphological activation in both cell lines.
Conclusions
Shock wave trauma by the Flyer Plate method caused an inflammatory response and morphological signs of activation in two macrophage cell lines, while iNOS induction appeared to require humoral signaling by LPS/IFN-ɣ. Our findings indicated that direct energy transfer by trauma can activate macrophages directly without humoral mediators, which comprises a novel activation mechanism of macrophages.












Similar content being viewed by others
References
Bains M, Hall ED (2011) Antioxidant therapies in traumatic brain and spinal cord injury. Biochim Biophys Acta 1822(5):675–684
Baskurt OK, Ulker P, Meiselman HJ (2011) Nitric oxide, erythrocytes and exercise. Clin Hemorheol Microcirc 49:175–181
Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A 87:1620–1624
Bogdan C (2001) Nitric oxide and the immune response. Nat Immunol 2:907–916
Brøchner AC, Toft P (2009) Pathophysiology of the systemic inflammatory response after major accidental trauma. Scand J Trauma Resusc Emerg Med 17:43
Chavko M, Watanabe T, Adeeb S, Lankasky J, Ahlers ST, McCarron RM (2011) Relationship between orientation to a blast and pressure wave propagation inside the rat brain. J Neurosci Methods 195:61–66
Chopra J, Joist JH, Webster RO (1987) Loss of 51chromium, lactate dehydrogenase, and 111indium as indicators of endothelial cell injury. Lab Invest J Tech Methods Pathol 57:578–584
Delacrétaz G, Rink K, Pittomvils G, Lafaut JP, Vandeursen H, Boving R (1995) Importance of the implosion of ESWL-induced cavitation bubbles. Ultrasound Med Biol 21:97–103
Feuerstein GZ, Wang X, Barone FC (1998) The role of cytokines in the neuropathology of stroke and neurotrauma. Neuroimmunomodulation 5:143–159
Floyd RA (1999) Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med 222:236–245
Gordon S, Martinez FO (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32:593–604
Griscavage JM, Rogers NE, Sherman MP, Ignarro LJ (1993) Inducible nitric-oxide synthase from a rat alveolar macrophage cell-line is inhibited by nitric oxide. J Immunol 151:6329–6337
Gunther M, Al Nimer F, Gahm C, Piehl F, Mathiesen T (2012) iNOS-mediated secondary inflammatory response differs between rat strains following experimental brain contusion. Acta Neurochir (Wien) 154(4):689–697
Huang DW, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57
Janzon B, Seeman T (1985) Muscle devitalization in high-energy missile wounds, and its dependence on energy transfer. J Trauma 25:138–144
Kumaria A, Tolias CM (2008) In vitro models of neurotrauma. Br J Neurosurg 22:200–206
Lu J, Goh SJ, Tng PY, Deng YY, Ling EA, Moochhala S (2009) Systemic inflammatory response following acute traumatic brain injury. Front Biosci 14:3795–3813
Lumeng CN, Bodzin JL, Saltiel AR (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117:175–184
MacMicking J, Xie QW, Nathan C (1997) Nitric oxide and macrophage function. Annu Rev Immunol 15:323–350
Marklund N, Bakshi A, Castelbuono DJ, Conte V, McIntosh TK (2006) Evaluation of pharmacological treatment strategies in traumatic brain injury. Curr Pharm Des 12:1645–1680
Miljkovic D, Trajkovic V (2004) Inducible nitric oxide synthase activation by interleukin-17. Cytokine Growth Factor Rev 15:21–32
Morrison B 3rd, Saatman KE, Meaney DF, McIntosh TK (1998) In vitro central nervous system models of mechanically induced trauma: a review. J Neurotrauma 15:911–928
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969
Schouten JW (2007) Neuroprotection in traumatic brain injury: a complex struggle against the biology of nature. Curr Opin Crit Care 13:134–142
Sonden A, Johansson AS, Palmblad J, Kjellstrom BT (2006) Proinflammatory reaction and cytoskeletal alterations in endothelial cells after shock wave exposure. J Invest Med Off Publ Am Fed Clin Res 54:262–271
Sonden A, Svensson B, Roman N, Brismar B, Palmblad J, Kjellstrom BT (2002) Mechanisms of shock wave induced endothelial cell injury. Lasers Surg Med 31:233–241
Sonden A, Svensson B, Roman N, Ostmark H, Brismar B, Palmblad J, Kjellstrom BT (2000) Laser-induced shock wave endothelial cell injury. Lasers Surg Med 26:364–375
Stuehr DJ, Marletta MA (1987) Synthesis of nitrite and nitrate in murine macrophage cell-lines. Cancer Res 47:5590–5594
Tsikas D (2007) Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: appraisal of the Griess reaction in the l-arginine/nitric oxide area of research. J Chromatogr B Anal Technol Biomed Life Sci 851:51–70
Wada K, Chatzipanteli K, Busto R, Dietrich WD (1998) Role of nitric oxide in traumatic brain injury in the rat. J Neurosurg 89:807–818
Acknowledgments
The authors would like to thank Elisabeth Malm for excellent technical assistance. We would also like to thank the core facility at Novum, BEA, Bioinformatics and Expression Analysis, which is supported by the board of research at the Karolinska Institute and the research committee at the Karolinska Hospital. This study was funded by ALF Stockholms Läns Landsting and The Swedish Defense Agency.
Conflict of interest
All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
Although the central nervous system (CNS) is considered somehow privileged under the immunologic point of view and poorly influenced by the inflammatory processes, a large body of data suggests a local inflammatory response after traumatic CNS injuries and indicate that aspects of this response to injury amplify the secondary damage [1, 2]. The cardinal features of inflammation, namely infiltration of inflammatory cells (neutrophils, microglia/macrophage, and lymphocytes), release of inflammatory mediators and activation of endothelial cells leading to increased vascular permeability, edema formation, blood–brain cord barrier disjunction and tissue destruction, have extensively been characterized in animal traumatic brain injury models.
The inflammatory response in the central nervous system after trauma may be initiated by neutrophils that infiltrate the lesion site. They can be found in the injury site 3-4 h after injury. The prevalence of neutrophils reaches a maximum 1-3 days after injury and remains significantly increased for up to 10 days. Neutrophils may release a number of substances such as reactive oxygen species, reactive nitrogen radicals, cytokines, chemokines, and a variety of enzymes.
The infiltration of leukocytes into the central nervous system is mediated by specific adhesion molecules present on both endothelial cells and leukocytes. Those molecules, vascular cells adhesion molecules (VCAMs) and inflammatory cells adhesion molecules (ICAMs), may facilitate the cell-to-cell interactions among endothelial, inflammatory, and nervous system cells. Furthermore, the neutrophils’ accumulation and activity are modulated by a number of cytokines, such as TNF-α, IL-1, and interleukin-6 (IL-6).
This study confirms some aspects of this process. The authors’ findings support the assumption that macrophages/microglia play a pivotal role in TBI secondary damage, but suggest that these cells produce toxic substances such as nitric oxide, only if stimulated by cytokines. These cytokines, at least in the early stage, could be delivered by resident cells including mechanically activated microglia or from few neutrophils present in tissue after injury.
References
1. Conti A, Cardali S, Genovese T, Di Paola R, La Rosa G (2003) Role of inflammation in the secondary injury following experimental spinal cord trauma. J Neurosurg Sci 47:89-94
2. Conti A, Miscusi M, Cardali S, Germano A, Suzuki H, Cuzzocrea S, Tomasello F (2007) Nitric oxide in the injured spinal cord: synthases cross-talk, oxidative stress and inflammation. Brain Res Rev 54:205-218
Alfredo Conti
Messina, Italy
Rights and permissions
About this article
Cite this article
Günther, M., Plantman, S., Gahm, C. et al. Shock wave trauma leads to inflammatory response and morphological activation in macrophage cell lines, but does not induce iNOS or NO synthesis. Acta Neurochir 156, 2365–2378 (2014). https://doi.org/10.1007/s00701-014-2243-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-014-2243-1