Abstract
Background
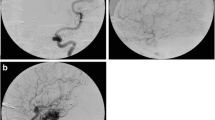
The reported AVMs obliteration rate after Gamma Knife radiosurgery (GKS) ranges from 70 to 94 %. The objective of the present study was to assess prognostic factors predictive for cerebral AVMs obliteration in 127 patients who underwent GKS.
Methods
The AVMs were classified according to the Spetzler-Martin classification. Twenty-one cases (16.5 %) were classified as grade I, 46 cases (36.2 %) as grade II, 51 cases (40.1 %) as grade III, and nine cases (7.1 %) as grade IV–V. The AVMs were deeply located in 16.5 % of patients. The peripheral prescription dose ranged from 16 to 30 Gy (mean 22.3 Gy). The AVMs volume ranged from 0.1 to 13 cc (mean 2.7 cc).
Results
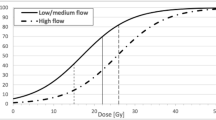
In 72 patients out of the 104 (69.2 %) with a radiological follow-up, MRI showed the AVM obliteration; in 54 cases (60 %) out of the 90 that performed a DSA, a complete AVM obliteration was achieved (average closure time 48.5 months). The volume of the nidus (p = 0.001), the prescription dose (p = 0.004), the 2002 Pollock–Flickinger classification (p = 0.031), and their 2008 revised classification (p = 0.025) were found to be statistically significant in predicting the probability of AVM closure. In the multivariate analysis, only the prescription dose was found to be an independent prognostic factor (p = 0.009) for AVM obliteration.
Conclusions
The volume of the nidus and the prescription dose significantly influence the outcome of radiosurgical treatment. The Pollock–Flickinger classification was found to be a reliable scoring system in predicting the AVM closure and an important tool for selection of patients candidate for GKS.


Similar content being viewed by others
References
Andrade-Souza YM, Zadeh G, Ramani M, Scora D, Tsao MN, Schwartz ML (2005) Testing the radiosurgery-based arteriovenous malformation score and the modified Spetzler-Martin grading system to predict radiosurgical outcome. J Neurosurg 103:642–648
Andrade-Souza YM, Zadeh G, Scora D, Tsao MN, Schwartz ML (2005) Radiosurgery for basal ganglia, internal capsule, and thalamus arteriovenous malformation: clinical outcome. Neurosurgery 56:56–63, discussion 63–54
Bamford JM, Sandercock PA, Warlow CP, Slattery J (1989) Interobserver agreement for the assessment of handicap in stroke patients. Stroke 20:828
Berman MF, Sciacca RR, Pile-Spellman J, Stapf C, Connolly ES Jr, Mohr JP, Young WL (2000) The epidemiology of brain arteriovenous malformations. Neurosurgery 47:389–396
Betti OO (1987) Treatment of arteriovenous malformations with the linear accelerator. Appl Neurophysiol 50:262
Brown RD Jr, Wiebers DO, Torner JC, O’Fallon WM (1996) Frequency of intracranial hemorrhage as a presenting symptom and subtype analysis: a population-based study of intracranial vascular malformations in Olmsted Country, Minnesota. J Neurosurg 85:29–32
Castel JP, Kantor G (2001) Postoperative morbidity and mortality after microsurgical exclusion of cerebral arteriovenous malformations. Current data and analysis of recent literature. Neurochirurgie 47:369–383
Cohen-Gadol AA, Pollock BE (2006) Radiosurgery for arteriovenous malformations in children. J Neurosurg 104:388–391
Colombo F, Pozza F, Chierego G, Casentini L, De Luca G, Francescon P (1994) Linear accelerator radiosurgery of cerebral arteriovenous malformations: an update. Neurosurgery 34:14–20, discussion 20–11
Crawford PM, West CR, Chadwick DW, Shaw MD (1986) Arteriovenous malformations of the brain: natural history in unoperated patients. J Neurol Neurosurg Psychiatry 49:1–10
Deruty R, Pelissou-Guyotat I, Mottolese C, Amat D, Bascoulergue Y, Turjman F, Gerard GP (1996) Therapeutic risk in multidisciplinary approach of cerebral arteriovenous malformations. Neurochirurgie 42:35–43
Flickinger JC, Kondziolka D, Pollock BE, Maitz AH, Lunsford LD (1997) Complications from arteriovenous malformation radiosurgery: multivariate analysis and risk modeling. Int J Radiat Oncol Biol Phys 38:485–490
Friedman WA, Blatt DL, Bova FJ, Buatti JM, Mendenhall WM, Kubilis PS (1996) The risk of hemorrhage after radiosurgery for arteriovenous malformations. J Neurosurg 84:912–919
Friedman WA, Bova FJ, Bollampally S, Bradshaw P (2003) Analysis of factors predictive of success or complications in arteriovenous malformation radiosurgery. Neurosurgery 52:296–307, discussion 307–298
Friedman WA, Bova FJ, Mendenhall WM (1995) Linear accelerator radiosurgery for arteriovenous malformations: the relationship of size to outcome. J Neurosurg 82:180–189
Graf CJ, Perret GE, Torner JC (1983) Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg 58:331–337
Hadjipanayis CG, Levy EI, Niranjan A, Firlik AD, Kondziolka D, Flickinger JC, Lunsford LD (2001) Stereotactic radiosurgery for motor cortex region arteriovenous malformations. Neurosurgery 48:70–76, discussion 76–77
Hladky JP, Lejeune JP, Blond S, Pruvo JP, Dhellemmes P (1994) Cerebral arteriovenous malformations in children: report on 62 cases. Childs Nerv Syst 10:328–333
Itoyama Y, Uemura S, Ushio Y, Kuratsu J, Nonaka N, Wada H, Sano Y, Fukumura A, Yoshida K, Yano T (1989) Natural course of unoperated intracranial arteriovenous malformations: study of 50 cases. J Neurosurg 71:805–809
Karlsson B, Lindquist C, Johansson A, Steiner L (1997) Annual risk for the first hemorrhage from untreated cerebral arteriovenous malformations. Minim Invasive Neurosurg 40:40–46
Karlsson B, Lindquist C, Steiner L (1996) Effect of Gamma Knife surgery on the risk of rupture prior to AVM obliteration. Minim Invasive Neurosurg 39:21–27
Kenny BG, Hitchcock ER, Kitchen G, Dalton AE, Yates DA, Chavda SV (1992) Stereotactic linac radiosurgery for arteriovenous malformations. J Neurol Neurosurg Psychiatry 55:590–593
Kjellberg RN, Hanamura T, Davis KR, Lyons SL, Adams RD (1983) Bragg-peak proton-beam therapy for arteriovenous malformations of the brain. N Engl J Med 309:269–274
Lawton MT (2003) Spetzler-Martin Grade III arteriovenous malformations: surgical results and a modification of the grading scale. Neurosurgery 52:740–748, discussion 748–749
Lawton MT, Hamilton MG, Spetzler RF (1995) Multimodality treatment of deep arteriovenous malformations: thalamus, basal ganglia, and brain stem. Neurosurgery 37:29–35, discussion 35–26
Levy EI, Niranjan A, Thompson TP, Scarrow AM, Kondziolka D, Flickinger JC, Lunsford LD (2000) Radiosurgery for childhood intracranial arteriovenous malformations. Neurosurgery 47:834–841, discussion 841–832
Loeffler JS, Alexander E 3rd, Siddon RL, Saunders WM, Coleman CN, Winston KR (1989) Stereotactic radiosurgery for intracranial arteriovenous malformations using a standard linear accelerator. Int J Radiat Oncol Biol Phys 17:673–677
Lunsford LD, Kondziolka D, Flickinger JC, Bissonette DJ, Jungreis CA, Maitz AH, Horton JA, Coffey RG (1991) Stereotactic radiosurgery for arteriovenous malformations of the brain. J Neurosurg 75:512–524
Menovsky T, van Overbeeke JJ (1997) Cerebral arteriovenous malformations in childhood: state of the art with special reference to treatment. Eur J Pediatr 156:741–746
Miyawaki L, Dowd C, Wara W, Goldsmith B, Albright N, Gutin P, Halbach V, Hieshima G, Higashida R, Lulu B, Pitts L, Schell M, Smith V, Weaver K, Wilson C, Larson D (1999) Five-year results of LINAC radiosurgery for arteriovenous malformations: outcome for large AVMS. Int J Radiat Oncol Biol Phys 44:1089–1106
Morgan MK, Johnston IH, Hallinan JM, Weber NC (1993) Complications of surgery for arteriovenous malformations of the brain. J Neurosurg 78:176–182
Morgan MK, Sekhon LH, Finfer S, Grinnell V (1999) Delayed neurological deterioration following resection of arteriovenous malformations of the brain. J Neurosurg 90:695–701
Mori K, Murata T, Hashimoto N, Handa H (1980) Clinical analysis of arteriovenous malformations in children. Childs Brain 6:13–25
Pan DH, Guo WY, Chung WY, Shiau CY, Chang YC, Wang LW (2000) Gamma knife radiosurgery as a single treatment modality for large cerebral arteriovenous malformations. J Neurosurg 93(Suppl 3):113–119
Pan HC, Sheehan J, Stroila M, Steiner M, Steiner L (2005) Late cyst formation following gamma knife surgery of arteriovenous malformations. J Neurosurg 102(Suppl):124–127
Paulsen RD, Steinberg GK, Norbash AM, Marcellus ML, Marks MP (1999) Embolization of basal ganglia and thalamic arteriovenous malformations. Neurosurgery 44:991–996, discussion 996–997
Perret G, Nishioka H (1966) Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. IV. Cerebral angiography. An analysis of the diagnostic value and complications of carotid and vertebral angiography in 5,484 patients. J Neurosurg 25:98–114
Pikus HJ, Beach ML, Harbaugh RE (1998) Microsurgical treatment of arteriovenous malformations: analysis and comparison with stereotactic radiosurgery. J Neurosurg 88:641–646
Pollock BE, Flickinger JC (2002) A proposed radiosurgery-based grading system for arteriovenous malformations. J Neurosurg 96:79–85
Pollock BE, Flickinger JC (2008) Modification of the radiosurgery-based arteriovenous malformation grading system. Neurosurgery 63:239–243, discussion 243
Pollock BE, Flickinger JC, Lunsford LD, Bissonette DJ, Kondziolka D (1996) Factors that predict the bleeding risk of cerebral arteriovenous malformations. Stroke 27:1–6
Pollock BE, Flickinger JC, Lunsford LD, Bissonette DJ, Kondziolka D (1996) Hemorrhage risk after stereotactic radiosurgery of cerebral arteriovenous malformations. Neurosurgery 38:652–659, discussion 659–661
Pollock BE, Flickinger JC, Lunsford LD, Maitz A, Kondziolka D (1998) Factors associated with successful arteriovenous malformation radiosurgery. Neurosurgery 42:1239–1244, discussion 1244–1237
Pollock BE, Gorman DA, Brown PD (2004) Radiosurgery for arteriovenous malformations of the basal ganglia, thalamus, and brainstem. J Neurosurg 100:210–214
Pollock BE, Lunsford LD, Kondziolka D, Maitz A, Flickinger JC (1994) Patient outcomes after stereotactic radiosurgery for “operable” arteriovenous malformations. Neurosurgery 35:1–7, discussion 7–8
Schaller C, Schramm J (1997) Microsurgical results for small arteriovenous malformations accessible for radiosurgical or embolization treatment. Neurosurgery 40:664–672, discussion 672–664
Schlienger M, Atlan D, Lefkopoulos D, Merienne L, Touboul E, Missir O, Nataf F, Mammar H, Platoni K, Grandjean P, Foulquier JN, Huart J, Oppenheim C, Meder JF, Houdart E, Merland JJ (2000) Linac radiosurgery for cerebral arteriovenous malformations: results in 169 patients. Int J Radiat Oncol Biol Phys 46:1135–1142
Sebag-Montefiore DJ, Doughty D, Biggs D, Plowman PN (1995) Stereotactic multiple arc radiotherapy. I. Vascular malformations of the brain: an analysis of the first 108 patients. Br J Neurosurg 9:441–452
Spetzler RF, Martin NA (1986) A proposed grading system for arteriovenous malformations. J Neurosurg 65:476–483
Steiner L, Lindquist C, Adler JR, Torner JC, Alves W, Steiner M (1992) Clinical outcome of radiosurgery for cerebral arteriovenous malformations. J Neurosurg 77:1–8
Zabel-du Bois A, Milker-Zabel S, Huber P, Schlegel W, Debus J (2006) Pediatric cerebral arteriovenous malformations: the role of stereotactic linac-based radiosurgery. Int J Radiat Oncol Biol Phys 65:1206–1211
Zabel-du Bois A, Milker-Zabel S, Huber P, Schlegel W, Debus J (2006) Stereotactic linac-based radiosurgery in the treatment of cerebral arteriovenous malformations located deep, involving corpus callosum, motor cortex, or brainstem. Int J Radiat Oncol Biol Phys 64:1044–1048
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
Radiosurgery of AVMs is still a challenge to the radio surgical community, with results ranging from no obliteration to obliteration to the development of adverse radiation effects. This explains the need for predictors of outcome in order to properly select patients who may benefit from radiosurgery. Previous studies have identified nidus volume, patient age, location, venous drainage pattern, and previous embolization as predictors of outcome. Various AVM grading systems relevant to radiosurgery have been based on combinations of those parameters. The authors present a retrospective review of their own data searching for predictors of obliteration. In this study, nidus volume, radiation dose, and AVM classification according to Pollock-Flickinger were the sole predictors of AVM obliteration following first-time, single-stage Gamma Knife radiosurgery. Nidus volume was the only independent predictor of AVM obliteration in this study. This is not surprising, since radiation dose depends mainly on nidus volume provided that radiation dose follows international standards. The authors have identified a cut-off nidus volume separating obliterated from non-obliterated cases. Nidus volumes of up to 3 cc were highly more likely to be associated with AVM obliteration than larger nidus volumes. In two older studies, the probability of obliteration was also volume dependent with smaller volumes being more likely to obliterate. According to the literature and the findings of this study, there seem to be three volume ranges of the AVM nidus relevant for radiosurgery: <3 cc, 3–10 cc, and >10 cc. An AVM nidus of <3 cc seems to respond with a high obliteration rate to single-stage radiosurgery, an AVM nidus of >10 cc seems to respond significantly less likely with obliteration, and the obliteration rate of an AVM nidus of 3–10 cc seems to be somewhere in between. This should be kept in mind when selecting patients with AVMs for radiosurgery. This study contributes to our understanding of radio surgical treatment of AVMs and to the goal of minimizing individual patients’ risks and optimizing treatment results.
Thomas Mindermann
Zurich, Switzerland
Rights and permissions
About this article
Cite this article
Franzin, A., Snider, S., Boari, N. et al. Evaluation of prognostic factors as predictor of AVMS obliteration after Gamma Knife radiosurgery. Acta Neurochir 155, 619–626 (2013). https://doi.org/10.1007/s00701-013-1631-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-013-1631-2