Abstract
Background
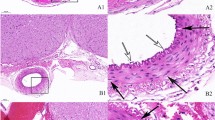
After subarachnoid hemorrhage (SAH), the formation of subarachnoid clots and their associated resolution may be involved in the development of chronic cerebral vasospasm. To dissolve and wash out the subarachnoid clot is one of the therapeutic strategies for prevention of cerebral vasospasm.
Objective
We investigated the effect of recombinant streptokinase (r-SK), a synthetic plasminogen activator, which is added to degenerate oxyhemoglobin, one of the strongest spasmogenic substances. The efficacy and safety of this therapy concerning the development of chronic cerebral vasospasm were evaluated in a swine model.
Methods
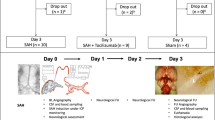
Eighteen healthy porcine subjects were used. Each was randomly assigned to one of three groups: saline control (A), SAH treated with saline (B), and r-SK injection into the cisterna magna (C). SAH was produced by introduction of blood clots into the cisterna magna on each of 2 days in all subjects in groups B and C. At 24-h post-SAH, a one-time dosage of 15 mg of r-SK was administered to those subjects randomized to group C. Continuous drainage was applied in all three groups. Vessel diameter was evaluated by angiography before the induction of SAH and at day 7 following SAH.
Results
The pre- and post-SAH angiographs of subjects in group A determined no significant difference in mean vessel caliber. In group B, pre- and post-SAH angiography indicated significant (p < 0.05) reductions of the mean vessel caliber of the right internal carotid artery (ICA) and basilar artery (BA) compared with the baseline values before SAH. In the r-SK treated group, the mean percent reduction in vessel caliber of the right ICA and BA on day 7 angiograms showed no significant difference compared with the baseline value before SAH.
Conclusions
Chronic cerebral vasospasm was inhibited in the animals to whom r-SK was administered for 1 day after double administration of blood clots to the cisterna magna to induce SAH. The results suggest that the post-SAH presence of subarachnoid clots that contain oxyhemoglobin might be involved in the pathogenesis of vasospasm. Further degeneration of these clots by r-SK may have a promising effect for prevention of vasospasm.






Similar content being viewed by others
References
Amin-Hanjani S, Ogilvy CS, Barker FG 2nd (2004) Does intracisternal thrombolysis prevent vasospasm after aneurysmal subarachnoid hemorrhage? A meta-analysis. Neurosurgery 54:326–334
Barth M, Capelle HH, Weidauer S, Weiss C, Münch E, Thomé C, Luecke T, Schmiedek P, Kasuya H, Vajkoczy P (2007) Effect of nicardipine prolonged-release implants on cerebral vasospasm and clinical outcome after severe aneurismal subarachnoid hemorrhage: a prospective, randomized, double- blind phase IIa study. Stroke 38:330–336
Giraldez RR, Wiviott SD, Nicolau JC, Mohanavelu S, Morrow DA, Antman EM, Giugliano RP (2009) Streptokinase and enoxaparin as an alternative to fibrin-specific lytic-based regimens: an ExTRACT-TIMI 25 analysis. Drugs 30:1433–1443
Chyatte D (1990) Anti-inflammatory agents and cerebral vasospasm. Neurosurg Clin N Am 1:433–450
Crompton MR (1964) The pathogenesis of cerebral infarct following the rupture of cerebral berry aneurysm. Brain 87:491–510
Findlay JM, Weir BK, Gordon P, Grace M, Baughman R (1989) Safety and efficacy of intrathecal thrombolytic therapy in a primate model of cerebral vasospasm. Neurosurgery 24:491–498
Findlay JM, Weir BK, Steinke D, Tanabe T, Gordon P, Grace M (1988) Effect of intrathecal thrombolytic therapy on subarachnoid clot and chronic vasospasm in a primate model of SAH. J Neurosurg 69:723–735
Fisher CM, Kistler JP, Davis JM (1980) Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 6:1–9
Gallia GL, Tamargo RJ (2006) Leukocyte-endothelial cell interactions in chronic vasospasm after subarachnoid hemorrhage. Neurol Res 28:750–758
Handa Y, Weir BKA, Nosko M, Mosewich R, Tsuji T, Grace M (1987) The effect of timing of clot removal on chronic vasospasm in a primate model. J Neurosurg 67:558–564
Handa Y, Hayashi M, Takeuchi H, Kubota T, Kobayashi H, Kawano H (1992) Time course of cerebral autoregulation during chronic cerebral vasospasm after subarachnoid hemorrhage in primates. J Neurosurg 76:493–501
Handa Y, Kaneko M, Matuda T, Kobayashi H, Kubota T (1997) In vivo proton magnetic resonance spectroscopy for metabolic changes in brain during chronic cerebral vasospasm in primates. Neurosurgery 40:773–781
Handa Y, Kubota T, Tsuchida A, Kaneko M, Caner H, Kobayashi H, Kubota T (1993) Effect of systemic hypotension on cerebral energy metabolism during chronic cerebral vasospasm in primates. J Neurosurg 78:112–119
Harrod CG, Bendok BR, Batjer HH (2005) Prediction of cerebral vasospasm in patients presenting with aneurysmal subarachnoid hemorrhage: a review. Neurosurgery 56:633–654
Hop JW, Rinkel GJ, Algra A, van Gijn J (1997) Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke 28:660–664
Hänggi D, Liersch J, Turowski B, Yong M, Steiger HJ (2008) The effect of lumboventricular lavage and simultaneous low- frequency head- motion therapy after severe subarachnoid hemorrhage: results of a single center prospective Phase II trial. J Neurosurg 108:1192–1199
Macdonald RL, Weir BKA (1991) A review of hemoglobin and the pathogenesis of cerebral vasospasm. Stroke 22:971–982
Macdonald RL, Weir BK (2001) Cerebral Vasospasm, San Diego
Osaka K (1977) Prolonged vasospasm produced by the break-down products of erythrocytes. J Neurosurg 47:403–411
Rabinstein AA, Friedman JA, Nichols DA, Pichelmann MA, McClelland RL, Manno EM, Atkinson JL, Wijdicks EF (2004) Predictors of outcome after endovascular treatment of cerebral vasospasm. Am J Neuroradiol 25:1778–1782
Ramakrishna R, Sekhar LN, Ramanathan D, Temkin N, Hallam D, Ghodke BV, Kim LJ (2010) Intraventricular tissue plasminogen activator for the prevention of vasospasm and hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery 67:110–117
Suarez JI, Tarr RW, Selman WR (2006) Aneurysmal subarachnoid hemorrhage. N Engl J Med 354:387–396
Varelas PN, Rickert KL, Cusick J, Hacein-Bey L, Sinson G, Torbey M, Spanaki M, Gennarelli TA (2005) Intraventricular hemorrhage after aneurismal SAH: pilot study of treatment with intraventricular TPA. Neurosurgery 56:205–213
Vatter H, Weidauer S, Konczalla J, Dettmann E, Zimmermann M, Raabe A, Preibisch C, Zanella FE, Seifert V (2006) Time course in the development of cerebral vasospasm after experimental subarachnoid hemorrhage: clinical and neuroradiological assessment of the rat double hemorrhage model. Neurosurgery 58:1190–1197
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
To date, neurosurgeons have become quite adept at managing the consequences of intracranial bleeds—elevated intracranial pressures and mass effect; however, we remain frustrated by the difficulty intracranial bleeds present when removal is necessary. Large clots may be resected, but diffuse bleeds cannot be reasonably removed and so may remain for a patient's own body to slowly resorb, a process commonly taking weeks. The CLEAR trial recently showed us that intraventricular hemorrhages may be safely removed at an accelerated pace through the injection of thrombolytics (1). This has opened a new avenue for clinicians treating diffuse intracranial bleeds.
The authors have applied the use of thrombolytics in this instance to a porcine model of subarachnoid hemorrhage. Their results are encouraging, demonstrating a reduction of oxyhemaglobin and cerebral vasospasm with cisternal thrombolytic injections. It must be noted that the model used does not simulate aneurysmal rupture itself. Thrombolytics carry the frightening risk of exacerbating intracranial bleeding in actively or recently ruptured aneurysm patients, and this scenario is not addressed in this article's study. Nonetheless, the results are intriguing and will, hopefully, be expanded by future studies.
Markus J. Bookland,
Christopher Loftus
Philadelphia, PA
1. Morgan T, Awad I, Keyl P, et al. (2008) Preliminary report of the clot lysis evaluating accelerated resolution of intraventricular hemorrhage (CLEAR-IVH) clinical trial. Acta Neurochir Suppl 105:21720.
Rights and permissions
About this article
Cite this article
Xu, H., Chen, X., Qin, Z. et al. Effect of recombinant streptokinase on the development of chronic cerebral vasospasm after subarachnoid hemorrhage in a swine model. Acta Neurochir 153, 1333–1338 (2011). https://doi.org/10.1007/s00701-011-0954-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-011-0954-0