Abstract
Purpose
Radical oxygen species produced after injury counteracts antioxidant activity and frequently causes severe oxidative stress for the tissues. Alpha-lipoic acid is a powerful metabolic antioxidant with immunomodulatory effects which provides neuroprotection. The aim of this study is to investigate the neuroprotective and anti-apoptotic effects of alpha-lipoic acid on spinal cord ischemia–reperfusion.
Methods
Twenty-four adult, male, New Zealand rabbits were divided into sham (n = 8), control (n = 8), and treatment groups (n = 8). The abdominal aorta was clamped for 30 min by an aneurysm clip, approximately 1 cm below the renal artery and 1 cm above the iliac bifurcation in control and treatment groups. Only laparotomy was performed in the sham group. Twenty-five cubic centimeters of saline in control group and 100 mg/kg lipoic acid were administered intraperitoneally in the treatment group after closure of the incision. The animals were killed 48 h later. Spinal cord segments between L2 and S1 were harvested for analysis. Levels of nitric oxide, glutathione, malondialdehyde, advanced oxidation protein products, and superoxide dismutase were analyzed as markers of oxidative stress and inflammation. Caspase-3 activity was analyzed to detect the effect of lipoic acid on apoptosis.
Results
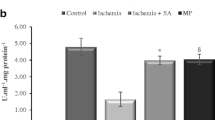
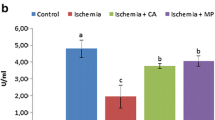
In all measured parameters of oxidative stress, administration of lipoic acid significantly demonstrated favorable effects. Both plasma and tissue levels of nitric oxide, glutathione, malondialdehyde, and advanced oxidation protein products significantly changed in favor of antioxidant activity. There was no significant difference between the plasma superoxide dismutase levels of the groups. Histopathological evaluation of the tissues also demonstrated significant decrease in cellular degeneration and infiltration parameters after lipoic acid administration. However, lipoic acid has no effect on caspase-3 activity.
Conclusions
Although further studies considering different dose regimens and time intervals are required, the results of the present study prove that alpha-lipoic acid has favorable effects on experimental spinal cord ischemia–reperfusion injury.



Similar content being viewed by others
References
Amar AP, Levy ML (1999) Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery 44:1027–1039, discussion 1039–1040
Aykac G, Uysal M, Yalcin AS, Kocak-Toker N, Sivas A, Oz H (1985) The effect of chronic ethanol ingestion on hepatic lipid peroxide, glutathione, glutathione peroxidase and glutathione transferase in rats. Toxicology 36:71–76
Azbill RD, Mu X, Bruce-Keller AJ, Mattson MP, Springer JE (1997) Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain Res 765:283–290
Baptiste DC, Fehlings MG (2006) Pharmacological approaches to repair the injured spinal cord. J Neurotrauma 23:318–334
Bilska A, Wlodek L (2005) Lipoic acid—the drug of the future? Pharmacol Rep 57:570–577
Bowes MP, Swanson S, Zivin JA (1996) The AMPA antagonist LY293558 improves functional neurological outcome following reversible spinal cord ischemia in rabbits. J Cereb Blood Flow Metab 16:967–972
Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings MG, Herr DL, Hitchon PW, Marshall LF, Nockels RP, Pascale V, Perot PL Jr, Piepmeier J, Sonntag VK, Wagner F, Wilberger JE, Winn HR, Young W (1998) Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg 89:699–706
Budwit-Novotny DA, McCarty KS, Cox EB, Soper JT, Mutch DG, Creasman WT, Flowers JL, McCarty KS Jr (1986) Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res 46:5419–5425
Cameron NE, Cotter MA, Horrobin DH, Tritschler HJ (1998) Effects of alpha-lipoic acid on neurovascular function in diabetic rats: interaction with essential fatty acids. Diabetologia 41:390–399
Cao X, Phillis JW (1995) The free radical scavenger, alpha-lipoic acid, protects against cerebral ischemia–reperfusion injury in gerbils. Free Radic Res 23:365–370
Casini AF, Ferrali M, Pompella A, Maellaro E, Comporti M (1986) Lipid peroxidation and cellular damage in extrahepatic tissues of bromobenzene-intoxicated mice. Am J Pathol 123:520–531
Chaudhary P, Marracci GH, Bourdette DN (2006) Lipoic acid inhibits expression of ICAM-1 and VCAM-1 by CNS endothelial cells and T cell migration into the spinal cord in experimental autoimmune encephalomyelitis. J Neuroimmunol 175:87–96
Christie SD, Comeau B, Myers T, Sadi D, Purdy M, Mendez I (2008) Duration of lipid peroxidation after acute spinal cord injury in rats and the effect of methylprednisolone. Neurosurg Focus 25:E5
Chronidou F, Apostolakis E, Papapostolou I, Grintzalis K, Georgiou CD, Koletsis EN, Karanikolas M, Papathanasopoulos P, Dougenis D (2009) Beneficial effect of the oxygen free radical scavenger amifostine (WR-2721) on spinal cord ischemia/reperfusion injury in rabbits. J Cardiothorac Surg 4:50
Conti A, Miscusi M, Cardali S, Germano A, Suzuki H, Cuzzocrea S, Tomasello F (2007) Nitric oxide in the injured spinal cord: synthases cross-talk, oxidative stress and inflammation. Brain Res Rev 54:205–218
Diaz-Ruiz A, Rios C, Duarte I, Correa D, Guizar-Sahagun G, Grijalva I, Madrazo I, Ibarra A (2000) Lipid peroxidation inhibition in spinal cord injury: cyclosporin-A vs methylprednisolone. NeuroReport 11:1765–1767
Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS (2001) Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol 24:254–264
Dumont RJ, Verma S, Okonkwo DO, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS (2001) Acute spinal cord injury, part II: contemporary pharmacotherapy. Clin Neuropharmacol 24:265–279
Erten SF, Kocak A, Ozdemir I, Aydemir S, Colak A, Reeder BS (2003) Protective effect of melatonin on experimental spinal cord ischemia. Spinal Cord 41:533–538
Genovese T, Cuzzocrea S (2008) Role of free radicals and poly(ADP-ribose)polymerase-1 in the development of spinal cord injury: new potential therapeutic targets. Curr Med Chem 15:477–487
Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D (2002) Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev 54:271–284
Gunasekar PG, Kanthasamy AG, Borowitz JL, Isom GE (1995) NMDA receptor activation produces concurrent generation of nitric oxide and reactive oxygen species: implication for cell death. J Neurochem 65:2016–2021
Hall ED (1993) The role of oxygen radicals in traumatic injury: clinical implications. J Emerg Med 11(Suppl 1):31–36
Hall ED, Springer JE (2004) Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx 1:80–100
Hall ED, Yonkers PA, Andrus PK, Cox JW, Anderson DK (1992) Biochemistry and pharmacology of lipid antioxidants in acute brain and spinal cord injury. J Neurotrauma 9(Suppl 2):S425–S442
Han D, Sen CK, Roy S, Kobayashi MS, Tritschler HJ, Packer L (1997) Protection against glutamate-induced cytotoxicity in C6 glial cells by thiol antioxidants. Am J Physiol 273:R1771–R1778
Hosaka N, Kimura S, Yamazaki A, Wang X, Denda H, Ito T, Hirano T, Endo N (2008) Significant correlation between cerebrospinal fluid nitric oxide concentrations and neurologic prognosis in incomplete cervical cord injury. Eur Spine J 17:281–286
Hulsebosch CE (2002) Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Educ 26:238–255
Ildan F, Polat S, Oner A, Isbir T, Gocer AI, Tap O, Kaya M, Karadayi A (1995) Effects of naloxone on sodium- and potassium-activated and magnesium-dependent adenosine-5'-triphosphatase activity and lipid peroxidation and early ultrastructural findings after experimental spinal cord injury. Neurosurgery 36:797–805
Kamencic H, Griebel RW, Lyon AW, Paterson PG, Juurlink BH (2001) Promoting glutathione synthesis after spinal cord trauma decreases secondary damage and promotes retention of function. FASEB J 15:243–250
Kaptanoglu E, Solaroglu I, Okutan O, Surucu HS, Akbiyik F, Beskonakli E (2004) Erythropoietin exerts neuroprotection after acute spinal cord injury in rats: effect on lipid peroxidation and early ultrastructural findings. Neurosurg Rev 27:113–120
Kaymaz M, Emmez H, Bukan N, Dursun A, Kurt G, Pasaoglu H, Pasaoglu A (2005) Effectiveness of FK506 on lipid peroxidation in the spinal cord following experimental traumatic injury. Spinal Cord 43:22–26
Kimura S, Hosaka N, Yuge I, Yamazaki A, Suda K, Taneichi H, Denda H, Endo N (2009) Cerebrospinal fluid concentrations of nitric oxide metabolites in spinal cord injury. Spine (Phila Pa 1976) 34:E645–E652
Kochhar A, Zivin JA, Lyden PD, Mazzarella V (1988) Glutamate antagonist therapy reduces neurologic deficits produced by focal central nervous system ischemia. Arch Neurol 45:148–153
Kowluru RA, Kern TS, Engerman RL (1997) Abnormalities of retinal metabolism in diabetes or experimental galactosemia. IV. Antioxidant defense system. Free Radic Biol Med 22:587–592
Kristian T, Siesjo BK (1998) Calcium in ischemic cell death. Stroke 29:705–718
Kurtel H, Granger DN, Tso P, Grisham MB (1992) Vulnerability of intestinal interstitial fluid to oxidant stress. Am J Physiol 263:G573–G578
Lang-Lazdunski L, Heurteaux C, Dupont H, Widmann C, Lazdunski M (2000) Prevention of ischemic spinal cord injury: comparative effects of magnesium sulfate and riluzole. J Vasc Surg 32:179–189
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lukacova N, Halat G, Chavko M, Marsala J (1996) Ischemia–reperfusion injury in the spinal cord of rabbits strongly enhances lipid peroxidation and modifies phospholipid profiles. Neurochem Res 21:869–873
McCarty KS Jr, Miller LS, Cox EB, Konrath J, McCarty KS Sr (1985) Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med 109:716–721
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62–71
Mitsui Y, Schmelzer JD, Zollman PJ, Mitsui M, Tritschler HJ, Low PA (1999) Alpha-lipoic acid provides neuroprotection from ischemia–reperfusion injury of peripheral nerve. J Neurol Sci 163:11–16
Mizuno Y, Ohta K (1986) Regional distributions of thiobarbituric acid-reactive products, activities of enzymes regulating the metabolism of oxygen free radicals, and some of the related enzymes in adult and aged rat brains. J Neurochem 46:1344–1352
Nagamatsu M, Nickander KK, Schmelzer JD, Raya A, Wittrock DA, Tritschler H, Low PA (1995) Lipoic acid improves nerve blood flow, reduces oxidative stress, and improves distal nerve conduction in experimental diabetic neuropathy. Diab Care 18:1160–1167
Oruckaptan HH, Ozisik P, Atilla P, Tuncel M, Kilinc K, Geyik PO, Basaran N, Yuksel E, Ozcan OE (2009) Systemic administration of interleukin-10 attenuates early ischemic response following spinal cord ischemia reperfusion injury in rats. J Surg Res 155:345–356
Packer L, Tritschler HJ, Wessel K (1997) Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radic Biol Med 22:359–378
Panigrahi M, Sadguna Y, Shivakumar BR, Kolluri SV, Roy S, Packer L, Ravindranath V (1996) alpha-Lipoic acid protects against reperfusion injury following cerebral ischemia in rats. Brain Res 717:184–188
Pekarkova I, Parara S, Holecek V, Stopka P, Trefil L, Racek J, Rokyta R (2001) Does exogenous melatonin influence the free radicals metabolism and pain sensation in rat? Physiol Res 50:595–602
Prehn JH, Karkoutly C, Nuglisch J, Peruche B, Krieglstein J (1992) Dihydrolipoate reduces neuronal injury after cerebral ischemia. J Cereb Blood Flow Metab 12:78–87
Qian H, Liu D (1997) The time course of malondialdehyde production following impact injury to rat spinal cord as measured by microdialysis and high pressure liquid chromatography. Neurochem Res 22:1231–1236
Rokkas CK, Helfrich LR Jr, Lobner DC, Choi DW, Kouchoukos NT (1994) Dextrorphan inhibits the release of excitatory amino acids during spinal cord ischemia. Ann Thorac Surg 58:312–319, discussion 319–320
Roy S, Packer L (1998) Redox regulation of cell functions by alpha-lipoate: biochemical and molecular aspects. Biofactors 8:17–21
Saunders RD, Dugan LL, Demediuk P, Means ED, Horrocks LA, Anderson DK (1987) Effects of methylprednisolone and the combination of alpha-tocopherol and selenium on arachidonic acid metabolism and lipid peroxidation in traumatized spinal cord tissue. J Neurochem 49:24–31
Schillace RV, Pisenti N, Pattamanuch N, Galligan S, Marracci GH, Bourdette DN, Carr DW (2007) Lipoic acid stimulates cAMP production in T lymphocytes and NK cells. Biochem Biophys Res Commun 354:259–264
Schmidley JW (1990) Free radicals in central nervous system ischemia. Stroke 21:1086–1090
Schonheit K, Gille L, Nohl H (1995) Effect of alpha-lipoic acid and dihydrolipoic acid on ischemia/reperfusion injury of the heart and heart mitochondria. Biochim Biophys Acta 1271:335–342
Serbinova E, Khwaja S, Reznick AZ, Packer L (1992) Thioctic acid protects against ischemia–reperfusion injury in the isolated perfused Langendorff heart. Free Radic Res Commun 17:49–58
Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM (2009) Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta 1790:1149–1160
Skvarilova M, Bulava A, Stejskal D, Adamovska S, Bartek J (2005) Increased level of advanced oxidation products (AOPP) as a marker of oxidative stress in patients with acute coronary syndrome. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 149:83–87
Smith AR, Shenvi SV, Widlansky M, Suh JH, Hagen TM (2004) Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr Med Chem 11:1135–1146
Sugawara T, Lewen A, Gasche Y, Yu F, Chan PH (2002) Overexpression of SOD1 protects vulnerable motor neurons after spinal cord injury by attenuating mitochondrial cytochrome c release. FASEB J 16:1997–1999
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Taskiran D, Kutay FZ, Sozmen E, Pogun S (1997) Sex differences in nitrite/nitrate levels and antioxidant defense in rat brain. NeuroReport 8:881–884
Tsai S-K, Hung L-M, Fu Y-T, Cheng H, Nien M-W, Liu H-Y, Zhang FB-Y, Huang S-S (2007) Resveratrol neuroprotective effects during focal cerebral ischemia injury via nitric oxide mechanism in rats. J Vasc Surg 46:346–353
Varija D, Kumar KP, Reddy KP, Reddy VK (2009) Prolonged constriction of sciatic nerve affecting oxidative stressors & antioxidant enzymes in rat. Indian J Med Res 129:587–592
Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B (1996) Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 49:1304–1313
Wolz P, Krieglstein J (1996) Neuroprotective effects of alpha-lipoic acid and its enantiomers demonstrated in rodent models of focal cerebral ischemia. Neuropharmacology 35:369–375
Xu M, Yip GW, Gan LT, Ng YK (2005) Distinct roles of oxidative stress and antioxidants in the nucleus dorsalis and red nucleus following spinal cord hemisection. Brain Res 1055:137–142
Yagi K (1982) Assay for serum lipid peroxide level and its clinical significance. In: Yagi K (ed) Lipid peroxides in biology and medicine. Academic, New York, pp 223–242
Yoshioka T, Kawada K, Shimada T, Mori M (1979) Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am J Obstet Gynecol 135:372–376
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
Currently, there is a tremendous research focus in animals and humans to improve the devastating effects of spinal cord injury. Some of the main strategies include avoidance of prehospital hypoxia and hypotension, early surgical decompression and spinal fracture stabilization, therapeutic hypothermia, neuroprotectants such as alpha-lipoic acid in this study, neurotrophic agents, cell transplantation, blocking myelin-based protein inhibitors, using neural scaffolds, anti-inflammatory agents, reducing glial scar formation, and new rehabilitation strategies.
H. Emmez and colleagues have reported a carefully conducted study using the antioxidant alpha-lipoic acid in an experimental spinal cord ischemia and reperfusion injury model in 24 rabbits. The animals were sacrificed 48 h after the injury was induced, and it was found that tissue and spinal cord tissue levels of various markers of oxidative stress were significantly improved and there was less cellular degeneration and inflammatory change in the affected spinal cord after intraperitoneal alpha-lipoic acid was administered shortly after 30 min of ischemia.
This experiment is a long way from the use of this agent in human spinal cord injury but is an important step along the way. There is a component of ischemia–reperfusion injury in human spinal cord injury, but there is also the mechanical injury. The ischemia–reperfusion injury model the authors have used is not therefore a complete model of human spinal cord injury. This antioxidant should be further investigated experimentally with different dose scales, administered at different time intervals after the trauma, for longer observation periods, and with different models such as weight drop or clamping that more closely replicate mechanical injury to the spinal cord or parts of it before sacrifice of the animals. Clearly there are ethical challenges to keeping animals alive longer after partial or complete spinal cord injury. We encourage the authors to continue their excellent research on this promising therapeutic agent.
Jeffrey V Rosenfeld
Departments of Neurosurgery and Surgery, The Alfred Hospital and Monash University, Melbourne, Australia.
Rights and permissions
About this article
Cite this article
Emmez, H., Yildirim, Z., Kale, A. et al. Anti-apoptotic and neuroprotective effects of alpha-lipoic acid on spinal cord ischemia–reperfusion injury in rabbits. Acta Neurochir 152, 1591–1601 (2010). https://doi.org/10.1007/s00701-010-0703-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-010-0703-9