Abstract
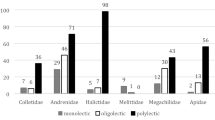
During the rainy season many species of Convolvulaceae bloom simultaneously in the Caatinga of northeast Brazil. In a Caatinga nature reserve we studied pollination and breeding systems of three sympatric species of Convolvulaceae, Ipomoea bahiensis, I. nil, and Merremia aegyptia, focusing on pollen partitioning among flower visitors and pollen flow. The study showed that only oligolectic bees collected pollen and that these species had different preferences among the three species of Convolvulaceae: pollen of Ipomoea bahiensis, the only self-incompatible species, was collected mainly by Melitoma segmentaria, M. osmioides, and Melitomella murihirta; pollen of I. nil by Lithurgus huberi; and that of Merremia aegyptia by Ancyloscelis apiformis and an undescribed species of this genus. Introduced honey bees visited only flowers of Merremia aegyptia, where they were extraordinarily frequent flower visitors. However, they discarded the pollen grains, which led to almost 50% pollen loss. No polylectic bee species compete for pollen with the oligolectic species. Partitioning of pollen diminishes competition for floral resources in this specialized plant–pollinator association.








Similar content being viewed by others
References
Aguiar CML, Martins CFM (1997) Abundância relativa, diversidade e fenologia de abelhas (Hymenoptera, Apoidea) na Caatinga, São João do Cariri, Paraíba, Brasil. Iheringia Sér Zool 83:151–163
Aguiar CML, Zanella FCV (2005) Estrutura da comunidade de abelhas (Hymenoptera: Apoidea: Apiformes) de uma área na margem do domínio caatinga (Itatim, BA). Neotrop Entomol 34:15–24
Alves-dos-Santos I (1999) Abelhas e plantas melíferas da mata atlântica, restinga e dunas do litoral norte do estado do Rio Grande do Sul, Brasil. Rev Bras Entomol 43:191–223
Alves-dos-Santos I (2000) Notes on bees of the tribe Emphorini. In: IV Encontro sobre Abelhas. Ribeirão Preto, Brazil, pp 211–215
Alves-dos-Santos I, Wittmann D (1999) The proboscis of the long-tongued Ancyloscelis bees (Anthophoridae/Apoidea), with remarks on flowers visits and pollen colleting with the mouth parts. J Kansas Entomol Soc 72:253–264
Alves-dos-Santos I, Wittmann D (2000) Legitimate pollination of the trimorphic flowers of Eichhornia azurea (Pontederiaceae) by Ancyloscelis gigas bees (Anthophoridae, Apoidea). Plant Syst Evol 223:127–137
Armbruster WS, Herzig AL (1984) Partitioning and sharing of pollinators by four sympatric species of Dalechampia (Euphorbiaceae) in Panama. Ann Missouri Bot Gard 71:1–16
Austin DF (1978) Morning glory bees and the Ipomoea pandurata complex (Hymenoptera: Anthophoridae). Proc Entomol Soc Wash 80(3):397–402
Austin DF, Huáman Z (1996) A synopsis of Ipomoea (Convolvulaceae) in the Americas. Taxon 45:3–38
Barcellos NDE, Paupitz J (1992) Caracterização da região do Seridó, RN. In: Projeto PNUD/FAO/IBAMA/BRA/87/007, Plano de manejo florestal para a região de Seridó do Rio Grande do Norte. pp 1–10
Cane JH, Sipes S (2006) Characterizing floral specialization by bees: analytical methods and a revised lexicon for oligolecty. In: Waser NM, Ollerton J (eds) Plant-pollinator interactions from specialization to generalization. pp 99–122
Caruso CM (1999) Pollination of Ipomopsis aggregata (Polemoniaceae): effects of intra- vs. interspecific competition. Am J Bot 86(5):663–668
Ducke A (1908) Contribuition à la connaissance de la faune hyménopterologique du nord-est du Brésil. II Hyménoptères récoltés dans I’État de Ceara em 1908. Revue d’Entomologie 27:57–87
Ducke A (1910) Explorações botânicas e entomológicas no Estado do Ceará. Rev Trim Inst Ceará 24:3–61
Duque JG (1973) O Nordeste e as Lavouras Xerófilas, 2nd edn. Banco do Nordeste, Fortaleza, Ceará
Gaglianone MC (2000) Behavior on flowers, structures involved in pollen transport and nesting biology of Perditomorpha brunerii and Cephalurgus anomalus (Hymenoptera: Colletidae, Andrenidae). Rev Biol Trop 48(1):89–99
Gentry AH (1974) Flowering phenology and diversity in tropical Bignoniaceae. Biotropica 6:64–68
Gimenes M (1997) Pollination bees and other visitors of Ludwigia elegans (Onagraceae) flowers at tropical sites in Brazil. Stud Neotrop Fauna Environm 32:81–88
Gimenes M (2002) Interactions between bees and Ludwigia elegans (Camb.) Hara (Onagraceae) flowers at different altitudes in São Paulo, Brazil. Rev Bras Zool 19(3):681–689
Giulietti AM, Conceição AA, Queiroz LP (2006) Diversidade e caracterização das fanerógamas do Semi-árido Brasileiro, vol 1. Associação Plantas do Nordeste, Recife.
IBAMA (1989) Caracterização da estação Ecológica de Seridó—RN. Natal RN Projeto PNUD/FAO/BRAZIL
Kearns AC, Inouye DW (1993) Techniques for pollination biologists. University of Colorado Press, Niwot, CO
Kiill LHP, Ranga NT (2000a) Biologia e sistema de reprodução de Jacquemontia multiflora (Choisy) Hallier f. (Convolvulaceae). Rev Bras Zool 23:37–43
Kiill LHP, Ranga NT (2000b) Biologia da polinização de Merremia aegyptia (L.) Urb. (Convolvulaceae) no Sertão de Pernambuco. Naturalia 25:149–158
Larkin LL, Neff JL, Simpson BB (2008) The evolution of a pollen diet: host choice and diet breadth of Andrena bees (Hymenoptera: Andrenidae). Apidologie 39:133–145
Larsson M, Franzén M (2007) Critical resource levels of pollen for the declining bee Andrena hattorfiana (Hymenoptera, Andrenidae). Biol Cons 134:405–414
Levin DA, Anderson WW (1970) Competition for pollinators between simultaneously flowering species. Am Nat 104:455–467
Linsley EG, MacSwain JW (1957) The nesting habits, flower relationships, and parasites of North American Species of Diadasina (Hymenoptera: Anthophoridae). Wasmann J Biol 15:199–233
Linsley EG, MacSwain JW, Smith RF (1956) Biological observations on Ptilothrix sumichrasti (Cresson) and some related groups of emphorine bees. Bull South Calif Acad Sci 55(2):83–101
Linsley EG, MacSwain JW, Michener CD (1980) Nesting biology and associates of Melitoma (Hymenoptera, Anthophoridae). Univ Calif Publ Entomol 90:1–45
Maimoni-Rodella RCS, Rodella AR (1992) Biologia floral de Ipomoea acuminata Roem. Et. Schult. (Convolvulaceae). Rev Bras Bot 15(2):129–133
Maiomani-Rodella RCS, Rodella RA, Amaral A Jr, Yanagizawa Y (1982) Polinização em Ipomoea cairica (L.) Sweet. (Convolvulaceae). Naturalia 7:167–172
Martins RP, Antonini Y (1994) The biology of Diadasina distincta (Holmberg, 1903) (Hymenoptera: Anthophoridae). Proc Entomol Soc Wash 96:553–560
Martins RP, Borges JC (1999) Use of Ludwigia (Onagraceae) pollen by a specialist bee, Diadasina distincta (Hymenoptera: Apidae), at a nesting site in Southeastern Brazil. Biotropica 31(3):530–534
McFarland JD, Kevan PG, Lane MA (1989) Pollination biology of Opuntia imbricata (Cactaceae) in southern Colorado. Can J Bot 67:24–28
Michener CD (2007) The bees of the world. Johns Hopkins University Press, Baltimore
Michener CD, McGinley RJ, Danforth BN (1994) The bee genera of North and Central America (Hymenoptera: Apoidea). Smithsonian Institution, Washington
Müller A, Diener S, Schnyder S, Stutz K, Sedivy C, Dorn S (2006) Quantitative pollen requirements of solitary bees: implications for bee conservation and the evolution of bee-flower relationships. Biol Cons 130:604–615
Osborn MM, Kevan PG, Lane MA (1988) Pollination biology of Opuntia polyacantha and Opuntia phaeacantha (Cactaceae) in Southern Colorado. Plant Syst Evol 159:85–94
Pinheiro M, Schlindwein C (1998) A câmara nectarífera de Ipomoea cairica (Convolvulaceae) e abelhas de glossa longa como polinizadores eficientes. Iheringia Ser Bot 5:3–16
Praz CJ, Müller A, Dorn S (2008) Specialized bees fail to develop on non-host pollen: do plants chemically protect their pollen? Ecology 89(3):795–804
Robertson C (1925) Heterotropic bees. Ecology 6:412–436
Rust RW (1980) The biology of Ptilothrix bombiformes (Hymenoptera: Anthophoridae). J Kansas Entomol Soc 53:427–436
Schlindwein C (1998) Frequent oligolecty characterizing a diverse bee-plant community in a xerophytic bushland of subtropical Brazil. Stud Neotrop Fauna Environm 33:46–59
Schlindwein C (2004) Are oligolectic bees always the most effective pollinators? In: Freitas BM, Pereira JO (eds) Solitary bees: conservation, rearing and management for pollination. Imprensa Universitária, Fortaleza, pp 231–240
Schlindwein C, Martins CF (2000) Competition between the oligolectic bee Ptilothrix plumata (Anthophoridae) and the flower closing beetle Pristimerus calcaratus (Curculionidae), for floral resources of Pavonia cancellata (Malvaceae). Plant Syst Evol 224:183–194
Schlindwein C, Wittmann D (1995) Specialized solitary bees as effective pollinators of South Brazilian species of Notocactus and Gymnocalcium. Bradleya 13:25–34
Schlindwein C, Wittmann D (1997) Stamen movements in flowers of Opuntia (Cactaceae) favour oligolectic pollinators. Plant Syst Evol 204:179–193
Schlindwein C, Wittmann D, Martins CF, Hamm A, Siqueira JA, Schiffler D, Machado IC (2005) Pollination of Campanula rapunculus L. (Campanulaceae): how much pollen flows into pollination and into reproduction of oligolectic pollinators? Plant Syst Evol 250:147–156
Schlindwein C, Pick RA, Martins CF (2009) Evaluation of oligolecty in the Brazilian bee Ptilothrix plumata (Hymenoptera, Apidae, Emphorini). Apidologie 40:106–116
Sedivy C, Praz CJ, Müller A, Widmer A, Dorn S (2008) Patterns of host-plant choice in bees of the genus Chelostoma: the constraint hypothesis of host-range evolution in bees. Evolution 62(10):2487–2507
Simão-Bianchini R (2002) Distribuição das espécies de Convolvulaceae na Caatinga. In: Sampaio EVS, Giulietti AM, Virginio J (eds) Vegetação e flora da Caatinga. Recife. Associação Plantas do Nordeste, APNE Centro Nordestino de Informações sobre Plantas. CNIP 176, pp 133–136
Sipes SD, Tepedino VJ (2005) Pollen-host specificity and evolutionary patterns of host switching in a clade of specialist bees (Apoidea: Diadasia). Biol J Linnean Soc 86:487–505
Snelling RR (1983) The North American species of the bee genus Lithurge (Hymenoptera: Megachilidae). Contrib Sci 343:1–11
Souza VC, Lorenzi H (2005) Botânica Sistemática: guia ilustrado para identificação das famílias de angiospermas da flora brasileira, baseado em APG II. Instituto Plantarum, São Paulo
Westrich P (1989) Die Wildbienen Baden-Württembergs. Teil 1: Lebensräume, Verhalten, Ökologie und Schutz. Ulmer Verlag, Stuttgart
Zanella FCV, Martins CF (2003) Abelhas da caatinga: biogeografia, ecologia e conservação. In: Leal IR, Tabarelli M, Silva JMC (eds) Ecologia e conservação da caatinga. Editora Universitária, UFPE, Recife, pp 75–134
Acknowledgments
We thank Fernando C.V. Zanella (UFCG) for his help in bee identification, Carlos Eduardo Nobre (UFPE) and Olaf Hermann Hendrik Mielke (UFPR) for identification of the butterflies, Rosângela Simão Bianchini for identification of the plants (Instituto de Botânica/SP), the staff of the Ecological Research Station ESEC-Seridó for logistical support, George Batista for botanical information about plants at the reserve, and IBAMA (Adson Borges Macedo) for permission to work in the nature reserve. Furthermore, we thank the members of the working group Plebeia-Ecologia de Abelhas e da Polinização for support in fieldwork, laboratory work, and for constructive discussions. The study was financially supported by the Brazilian Research Council-CNPq (471401/2006-4), Research Program in Biodiversity-PPBio Semi-árido, and grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) to R.A.P. and the Brazilian Research Council to C.S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pick, R.A., Schlindwein, C. Pollen partitioning of three species of Convolvulaceae among oligolectic bees in the Caatinga of Brazil. Plant Syst Evol 293, 147–159 (2011). https://doi.org/10.1007/s00606-011-0432-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-011-0432-4