Abstract
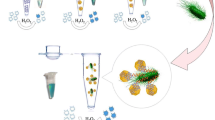
An unsophisticated fluorescence-enabled strategy is brought forward to process the highly sensitive fluorescence detection of Salmonella typhimurium (S. typhimurium) which based on polyethyleneimine (PEI)-templated silver/copper nanoclusters (Ag/CuNCs) (λ excitation = 334 nm and λ emission = 466 nm) with cryonase-assisted target recycling amplification. The Ag/CuNCs nanoclusters are synthesized as fluorescent materials due to their strong and stable fluorescence characteristics and are modified with S. typhimurium aptamers to form aptamer-Ag/CuNCs probes. The probes can be adsorbed on the surface of quenching agents-polydopamine nanospheres (PDANSs), thereby inducing fluorescence quenching of the probes. Once the aptamers are bound to the target, the aptamers/targets complexes are separated from the PDANSs surface, and the Ag/CuNCs recover the fluorescence signal. The released complexes will immediately be transformed into a substrate digested by cryonase (an enzyme that can digest all types of nucleic acids), and the released targets are bound to another aptamers to initiate the next round of cleavage. This reaction will be repeated continuously until all relevant aptamers are consumed and all Ag/CuNCs are released, resulting in a significant amplification of the fluorescence signal and improved sensitivity. Using Ag/CuNCs as fluorescent probes combined with cryonase-assisted amplification strategy, the fluorescence aptasensor is constructed with detection limits as low as 3.8 CFU mL−1, which is tenfold better than without the cryonase assistance. The method developed has been applied to milk, orange juice, chicken, and egg white samples with excellent selectivity and accuracy providing an approach for the early and rapid detection of S. typhimurium in food.
Graphical Abstract








Similar content being viewed by others
Data Availability
The data that has been used is confidential.
References
Punchihewage-Don AJ, Hawkins J, Adnan AM, Hashem F, Parveen S (2022) The outbreaks and prevalence of antimicrobial resistant Salmonella in poultry in the United States: An overview. Heliyon 8:e11571. https://doi.org/10.1016/j.heliyon.2022.e11571
Liu MY, Yue FL, Kong QQ, Liu ZL, Guo YM, Sun X (2022) Aptamers against pathogenic bacteria: selection strategies and apta-assay/aptasensor application for food safety. J Agric Food Chem 70:5477–5498. https://doi.org/10.1021/acs.jafc.2c01547
Courrol LC, Vallim MA (2021) Characterization of chicken meat contaminated with Salmonella by fluorescence spectroscopy. Spectrochim Acta A 261:119986. https://doi.org/10.1016/j.saa.2021.119986
Yang P, Wong C, Hash S, Fung F, Menon S (2018) Rapid detection of Salmonella spp. using magnetic resonance. J Food Safety 38:e12473. https://doi.org/10.1111/jfs.12473
Chen M, Pan L, Tu K (2021) A fluorescence biosensor for Salmonella typhimurium detection in food based on the nano-self-assembly of alendronic acid modified upconversion and gold nanoparticles. Anal Methods–UK 13:2415–2423. https://doi.org/10.1039/D1AY00493J
Wang XL, Niazi S, Huang YK, Sun WJ, Wu SJ, Duan N, Xu H, Wang ZP (2017) Homogeneous time-resolved FRET assay for the detection of Salmonella typhimurium using aptamer-modified NaYF 4: Ce/Tb nanoparticles and a fluorescent DNA label. Microchim Acta 184:4021–4027. https://doi.org/10.1007/s00604-017-2399-5
Lee KM, Runyon M, Herrman TJ, Phillips R, Hsieh J (2015) Review of Salmonella detection and identification methods: aspects of rapid emergency response and food safety. Food Control 47:264–276. https://doi.org/10.1016/j.foodcont.2014.07.011
Wan JJ, Zheng LP, Kong LY, Lu ZX, Yang T, Feng ZY, Lv FX, Meng FQ, Bie XM (2021) Development of a rapid detection method for real-time fluorescent quantitative PCR of Salmonella spp. and Salmonella Enteritidis in ready–to–eat fruits and vegetables. LWT 149:111837. https://doi.org/10.1016/j.lwt.2021.111837
Wang S, Zheng L, Cai G, Liu N, Liao M, Li Y, Zhang X, Lin J (2019) A microfluidic biosensor for online and sensitive detection of Salmonella typhimurium using fluorescence labeling and smartphone video processing. Biosens Bioelectron. 140:111333. https://doi.org/10.1016/j.bios.2019.111333
Rohde A, Hammerl JA, Boone I, Jansen W, Fohler S, Klein G, Dieckmann R, Dahouk SA (2017) Overview of validated alternative methods for the detection of foodborne bacterial pathogens. Trends Food Sci Tech 62:113–118. https://doi.org/10.1016/j.tifs.2017.02.006
Zhang XL, Xu JG, Chao Y, Li Y, Shang HJ, Chen W (2021) A short-and long-range fluorescence resonance energy transfer–cofunctionalized fluorescence quenching collapsar probe regulates amplified and accelerated detection of Salmonella. J Agric Food Chem 69:14294–14301. https://doi.org/10.1021/acs.jafc.1c05780
Yu S, Xu Q, Huang J, Yi B, Aguilar ZP, Xu H (2021) Rapid and sensitive detection of Salmonella in milk based on hybridization chain reaction and graphene oxide fluorescence platform. J Dairy Sci 104:12295–12302. https://doi.org/10.3168/jds.2021-20713
Tessaro L, Aquino A, de Almeida Rodrigues P, Joshi N, Ferrari RG, Conte-Junior CA (2022) Nucleic acid-based nanobiosensor (NAB) used for salmonella detection in foods: a systematic review. Nanomaterials 12:821. https://doi.org/10.3390/nano12050821
Sadanandan S, Ramkumar K, Pillai NP, Anuvinda P, Devika V, Ramanunni K, Sreejaya MM (2022) Biorecognition elements appended gold nanoparticle biosensors for the detection of food-borne pathogens-A review. Food Control 109510. https://doi.org/10.1016/j.foodcont.2022.109510
Singh P, Gupta R, Sinha M, Kumar R, Bhalla V (2016) MoS2 based digital response platform for aptamer based fluorescent detection of pathogens. Microchim Acta 183:1501–1506. https://doi.org/10.1007/s00604-016-1762-2
Sun X, Wang Y, Zhang L, Liu S, Zhang M, Wang J, Ning B, Peng Y, He J, Hu YG, Gao ZX (2020) CRISPR-Cas9 triggered two-step isothermal amplification method for E. coli O157: H7 detection based on a metal–organic framework platform. Anal Chem 92:3032–3041. https://doi.org/10.1021/acs.analchem.9b04162
Ding SY, Hu HL, Yue XL, Feng KW, Gao XY, Dong QL, Yang MQ, Tamer U, Huang GH, Zhang JS (2022) A fluorescent biosensor based on quantum dot–labeled streptavidin and poly–l–lysine for the rapid detection of Salmonella in milk. J Dairy Sci 105:2895–2907. https://doi.org/10.3168/jds.2021-21229
Yan J, Yang LP, Lin MF, Ma J, Lu XH, Lee PS (2013) Polydopamine spheres as active templates for convenient synthesis of various nanostructures. Small 9:596–603. https://doi.org/10.1002/smll.201201064
Yang JL, Song NZ, Jia Q (2019) Electrostatically controlled fluorometric assay for differently charged biotargets based on the use of silver/copper bimetallic nanoclusters modified with polyethyleneimine and graphene oxide. Microchim Acta 186:1–10. https://doi.org/10.1007/s00604-018-3179-6
Zhang BZ, Wei CY (2020) The sensitive detection of ATP and ADA based on turn-on fluorescent copper/silver nanoclusters. Anal Bioanal Chem 412:2529–2536. https://doi.org/10.1007/s00216-020-02476-0
Gong XY, Yu C, Zhang YC, Sun Y, Ye L, Li J (2019) Carbon nanoparticle-protected RNA aptasensor for amplified fluorescent determination of theophylline in serum based on nuclease-aided signal amplification. RSC Adv 9:33898–33902. https://doi.org/10.1039/c9ra06798a
Zhu L, Xu G, Song Q, Tang T, Wang X, Wei F, Hu Q (2016) Highly sensitive determination of dopamine by a turn-on fluorescent biosensor based on aptamer labeled carbon dots and nano–graphite. Sens Actuators B: Chem 231506–512. https://doi.org/10.1016/j.snb.2016.03.084
Ye YW, Zheng LB, Wu TT, Ding XW, Chen F, Yuan YY, Fan GC, Shen YZ (2020) Size-dependent modulation of polydopamine nanospheres on smart nanoprobes for detection of pathogenic bacteria at single-cell level and imaging-guided photothermal bactericidal activity. ACS Appl Mater Interfaces 12:35626–35637. https://doi.org/10.1021/acsami.0c07784
Lou YF, Peng YB, Luo XW, Yang ZM, Wang RF, Sun DW, Li LXY, Tan YY, Huang JH, Cui L (2019) A universal aptasensing platform based on cryonase–assisted signal amplification and graphene oxide induced quenching of the fluorescence of labeled nucleic acid probes: application to the detection of theophylline and ATP. Microchim Acta 186:1–9. https://doi.org/10.1007/s00604-019-3596-1
Huang XN, Shahzad SA, Li YX, Zhang YY, Sang LJ, Zhou HP, Jiang H, Kam-Wing Lo K, Yu C (2017) Silver nanoclusters capped silica nanoparticles as a ratiometric photoluminescence nanosensor for the selective detection of I− and S2−. Anal Chim Acta 988:74–80. https://doi.org/10.1016/j.aca.2017.07.056
Liu ZC, Zhang L, Song W, Liang RP, Qiu JD (2015) Cu nanoclusters-based ratiometric fluorescence probe for ratiometric and visualization detection of copper ions. Anal Chim Acta 895:95–103. https://doi.org/10.1016/j.aca.2015.09.002
Liu CY, Ding YQ, Li QJ, Lin YQ (2017) Photochemical synthesis of glutathione-stabilized silver nanoclusters for fluorometric determination of hydrogen peroxide. Microchim Acta 184:2497–2503. https://doi.org/10.1007/s00604-017-2302-4
Liu Q, Lai Q, Li N, Su XG (2018) Copper nanoclusters capped with tannic acid as a fluorescent probe for real-time determination of the activity of pyrophosphatase. Microchim Acta 185:1–7. https://doi.org/10.1007/s00604-017-2599-z
Xu SH, Nie YY, Jiang LP, Wang J, Xu GY, Wang W, Luo XL (2018) Polydopamine nanosphere/gold nanocluster (AuNC)-based nanoplatform for dual color simultaneous detection of multiple tumor-related microRNAs with DNase I assisted target recycling amplification. Anal Chem 90:4039–4045. https://doi.org/10.1021/acs.analchem.7b05253
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31872909, No.31772068).
Author information
Authors and Affiliations
Contributions
Shouyi Dou, Mengyue Liu, and Xia Sun designed the experiments. Shouyi Dou wrote the manuscript and performed the experiments. Falan Li and Yemin Guo provided writing assistance for the manuscript. Fengjuan Zhang, Baoxin Li, and Yuhao Zhang analyzed the data and drafted the manuscript. All authors have approved and read the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. The silver/copper bimetal nanoclusters combined with cryonase-assisted amplification are adopted to accomplish the highly sensitive detection of Salmonella typhimurium.
2. The aptamer-Ag/CuNCs fluorescent probes are successfully constructed for fluorescence resonance energy transfer to achieve Salmonella typhimurium rapid and highly sensitive detection.
3. The detection limit is as low as 3.8 CFU mL−1, which is 10 times lower than without the cryonase assistance.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dou, S., Liu, M., Zhang, F. et al. Silver/copper bimetallic nanoclusters integrating with cryonase-assisted target recycling amplification detection of Salmonella typhimurium. Microchim Acta 190, 403 (2023). https://doi.org/10.1007/s00604-023-05973-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05973-y