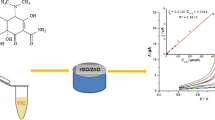
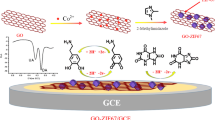
Abstract
Contributing to the development of sustainable electroanalytical chemistry, electrochemically reduced graphene oxide (ERGO) films obtained from residual graphite of discharged Zn-C batteries are proposed in this work. Graphite from the cathode of discarded Zn-C batteries was recovered and used in the synthesis of graphene oxide (GO) by the modified Hummer’s method. The quality of the synthesized GO was verified using different characterization methods (FT-IR, XRD, SEM, and TEM). GO films were deposited on a glassy carbon electrode (GCE) by the drop coating method and then electrochemically reduced by cathodic potential scanning using cyclic voltammetry. The electrochemical features of the ERGO films were investigated using the ferricyanide redox probe, as well as paracetamol (PAR) and hydroquinone (HQ) molecules as model analytes. From the cyclic voltammetry assays, enhanced heterogeneous electron transfer rate constants (k0) were observed for all redox systems studied. In analytical terms, the ERGO-based electrode showed higher analytical sensitivity than the bare and GO-modified GCE. Using differential pulse voltammetry, wide linear response ranges and limits of detection of 0.14 μmol L−1 and 0.65 μmol L−1 were achieved for PAR and HQ, respectively. Furthermore, the proposed sensor was successfully applied to the determination of PAR and HQ in synthetic urine and tap water samples (recoveries close to 100%). The outstanding electrochemical and analytical properties of the proposed ERGO films are added to the very low cost of the raw material, being presented as a green-based alternative for the development of electrochemical (bio)sensors with unsophisticated resources.
Graphical abstract










Similar content being viewed by others
References
Tran HP, Schaubroeck T, Swart P et al (2018) Recycling portable alkaline/ZnC batteries for a circular economy: An assessment of natural resource consumption from a life cycle and criticality perspective. Resour Conserv Recycl 135:265–278. https://doi.org/10.1016/j.resconrec.2017.08.018
Kumar N, Abubakar Sadique M, Khan R (2021) Electrochemical exfoliation of graphene quantum dots from waste dry cell battery for biosensor applications. Mater Lett 305:130829. https://doi.org/10.1016/j.matlet.2021.130829
Azam MG, Kabir MH, Shaikh MAA et al (2022) A rapid and efficient adsorptive removal of lead from water using graphene oxide prepared from waste dry cell battery. J Water Process Eng 46:102597. https://doi.org/10.1016/j.jwpe.2022.102597
da Silva AD, Paschoalino WJ, Damasceno JPV, Kubota LT (2020) Structure, Properties, and Electrochemical Sensing Applications of Graphene-Based Materials. Chem Electro Chem 7:4508–4525. https://doi.org/10.1002/celc.202001168
Ramya M, Senthil Kumar P, Rangasamy G et al (2022) A recent advancement on the applications of nanomaterials in electrochemical sensors and biosensors. Chemosphere 308:136416. https://doi.org/10.1016/j.chemosphere.2022.136416
Zhang L, Guo H, Xue R et al (2020) In-situ facile synthesis of flower shaped NiS2@regenerative graphene oxide derived from waste dry battery nano-composites for high-performance supercapacitors. J Energy Storage 31:101630. https://doi.org/10.1016/j.est.2020.101630
Bandi S, Ravuri S, Peshwe DR, Srivastav AK (2019) Graphene from discharged dry cell battery electrodes. J Hazard Mater 366:358–369. https://doi.org/10.1016/j.jhazmat.2018.12.005
Hummers WSJ, Offeman RE (1958) Preparation of Graphitic Oxide. J Am Chem Soc 80:1339. https://doi.org/10.1021/ja01539a017
Guo H-L, Wang X-F, Qian Q-Y et al (2009) A Green Approach to the Synthesis of Graphene Nanosheets. ACS Nano 3:2653–2659. https://doi.org/10.1021/nn900227d
Parham H, Zargar B (2001) Determination of isosorbide dinitrate in arterial plasma, synthetic serum and pharmaceutical formulations by linear sweep voltammetry on a gold electrode. Talanta 55:255–262. https://doi.org/10.1016/S0039-9140(01)00416-7
Stobinski L, Lesiak B, Malolepszy A et al (2014) Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J Electron Spectros Relat Phenomena 195:145–154. https://doi.org/10.1016/j.elspec.2014.07.003
Zhang L, Liang J, Huang Y et al (2009) Size-controlled synthesis of graphene oxide sheets on a large scale using chemical exfoliation. Carbon N Y 47:3365–3368. https://doi.org/10.1016/j.carbon.2009.07.045
Zhao W, Kido G, Hara K, Noguchi H (2014) Characterization of neutralized graphite oxide and its use in electric double layer capacitors. J Electroanal Chem 712:185–193. https://doi.org/10.1016/j.jelechem.2013.11.007
Marcano DC, Kosynkin DV, Berlin JM et al (2010) Improved Synthesis of Graphene Oxide. ACS Nano 4:4806–4814. https://doi.org/10.1021/nn1006368
Rabia M, Hadia NMA, Farid OM et al (2022) Poly(m-toluidine)/rolled graphene oxide nanocomposite photocathode for hydrogen generation from wastewater. Int J Energy Res 46:11943–11956. https://doi.org/10.1002/er.7963
Jankovský O, Jiříčková A, Luxa J et al (2017) Fast Synthesis of Highly Oxidized Graphene Oxide. ChemistrySelect 2:9000–9006. https://doi.org/10.1002/slct.201701784
Caridad JM, Rossella F, Bellani V et al (2011) Automated detection and characterization of graphene and few-layer graphite via Raman spectroscopy. J Raman Spectrosc 42:286–293. https://doi.org/10.1002/jrs.2739
Rattana T, Chaiyakun S, Witit-Anun N et al (2012) Preparation and characterization of graphene oxide nanosheets. Procedia Eng 32:759–764. https://doi.org/10.1016/j.proeng.2012.02.009
Ferrari AC, Meyer JC, Scardaci V et al (2006) Raman spectrum of graphene and graphene layers. Phys Rev Lett 97:1–4. https://doi.org/10.1103/PhysRevLett.97.187401
López-Díaz D, López Holgado M, García-Fierro JL, Velázquez MM (2017) Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J Phys Chem C 121:20489–20497. https://doi.org/10.1021/acs.jpcc.7b06236
Ambrosi A, Pumera M (2016) Electrochemically Exfoliated Graphene and Graphene Oxide for Energy Storage and Electrochemistry Applications. Chem – A Eur J 22:153–159. https://doi.org/10.1002/chem.201503110
Zhang Z, Yan J, Jin H, Yin J (2014) Tuning the reduction extent of electrochemically reduced graphene oxide electrode film to enhance its detection limit for voltammetric analysis. Electrochim Acta 139:232–237. https://doi.org/10.1016/j.electacta.2014.06.159
Raj MA, John SA (2013) Fabrication of Electrochemically Reduced Graphene Oxide Films on Glassy Carbon Electrode by Self-Assembly Method and Their Electrocatalytic Application. J Phys Chem C 117:4326–4335. https://doi.org/10.1021/jp400066z
Chen P, McCreery RL (1996) Control of Electron Transfer Kinetics at Glassy Carbon Electrodes by Specific Surface Modification. Anal Chem 68:3958–3965. https://doi.org/10.1021/ac960492r
Ambrosi A, Pumera M (2013) Precise Tuning of Surface Composition and Electron-Transfer Properties of Graphene Oxide Films through Electroreduction. Chem – A Eur J 19:4748–4753. https://doi.org/10.1002/chem.201204226
Ambrosi A, Chua CK, Latiff NM et al (2016) Graphene and its electrochemistry – an update. Chem Soc Rev 45:2458–2493. https://doi.org/10.1039/C6CS00136J
Moo JGS, Ambrosi A, Bonanni A, Pumera M (2012) Inherent Electrochemistry and Activation of Chemically Modified Graphenes for Electrochemical Applications. Chem – An Asian J 7:759–770. https://doi.org/10.1002/asia.201100852
Pumera M (2013) Electrochemistry of graphene, graphene oxide and other graphenoids: Review. Electrochem commun 36:14–18. https://doi.org/10.1016/j.elecom.2013.08.028
Bard AJ, Faulkner L (2001) Electrochemical Methods - Fundamental and Applications, 2nd edn, New York
Nicholson RS, Shain I (1964) Theory of Stationary Electrode Polarography. Single Scan and Cyclic Methods Applied to Reversible, Irreversible, and Kinetic Systems. Anal Chem 36:706–723. https://doi.org/10.1021/ac60210a007
Lavagnini I, Antiochia R, Magno F (2004) An Extended Method for the Practical Evaluation of the Standard Rate Constant from Cyclic Voltammetric Data. Electroanalysis 16:505–506. https://doi.org/10.1002/elan.200302851
Silva TA, Zanin H, May PW et al (2014) Electrochemical performance of porous diamond-like carbon electrodes for sensing hormones, neurotransmitters, and endocrine disruptors. ACS Appl Mater Interfaces 6. https://doi.org/10.1021/am505928j
Patel M, Bisht N, Prabhakar P et al (2023) Ternary nanocomposite-based smart sensor: Reduced graphene oxide/polydopamine/alanine nanocomposite for simultaneous electrochemical detection of Cd2+, Pb2+, Fe2+, and Cu2+ ions. Environ Res 221:115317. https://doi.org/10.1016/j.envres.2023.115317
Huang J, Qiu Z, Lin J et al (2023) Ultrasensitive determination of metronidazole using flower-like cobalt anchored on reduced graphene oxide nanocomposite electrochemical sensor. Microchem J 188:108444. https://doi.org/10.1016/j.microc.2023.108444
Naik TSSK, Swamy BEK, Ramamurthy PC, Chetankumar K (2020) Poly (L-leucine) modified carbon paste electrode as an electrochemical sensor for the detection of paracetamol in presence of folic acid. Mater Sci Energy Technol 3:626–632. https://doi.org/10.1016/j.mset.2020.07.003
Mangaiyarkarasi R, Premlatha S, Khan R et al (2020) Electrochemical performance of a new imidazolium ionic liquid crystal and carbon paste composite electrode for the sensitive detection of paracetamol. J Mol Liq 319:114255. https://doi.org/10.1016/j.molliq.2020.114255
Liu B, Guo H, Sun L et al (2022) Electrochemical sensor based on covalent organic frameworks/MWCNT for simultaneous detection of catechol and hydroquinone. Colloids Surfaces A Physicochem Eng Asp 639:128335. https://doi.org/10.1016/j.colsurfa.2022.128335
Yang X, He C, Lin W et al (2022) Electrochemical sensors for hydroquinone and catechol based on nano-flake graphite and activated carbon sensitive materials. Synth Met 287:117079. https://doi.org/10.1016/j.synthmet.2022.117079
Maciel CC, de Lima LF, Ferreira AL et al (2022) Development of a flexible and disposable electrochemical sensor based on poly (butylene adipate-co-terephthalate) and graphite for hydroquinone sensing. Sensors and Actuators Reports 4:100091. https://doi.org/10.1016/j.snr.2022.100091
Wang C, Li C, Wang F, Wang C (2006) Covalent Modification of Glassy Carbon Electrode with L-Cysteine for the Determination of Acetaminophen. Microchim Acta 155:365–371. https://doi.org/10.1007/s00604-006-0616-8
Peng J, Gao Z-N (2006) Influence of micelles on the electrochemical behaviors of catechol and hydroquinone and their simultaneous determination. Anal Bioanal Chem 384:1525–1532. https://doi.org/10.1007/s00216-006-0329-1
Manoj D, Rajendran S, Hoang TKA et al (2022) In-situ growth of 3D Cu-MOF on 1D halloysite nanotubes/reduced graphene oxide nanocomposite for simultaneous sensing of dopamine and paracetamol. J Ind Eng Chem 112:287–295. https://doi.org/10.1016/j.jiec.2022.05.022
Kader Mohiuddin A, Shamsuddin Ahmed M, Jeon S (2022) Palladium doped α-MnO2 nanorods on graphene as an electrochemical sensor for simultaneous determination of dopamine and paracetamol. Appl Surf Sci 578:152090. https://doi.org/10.1016/j.apsusc.2021.152090
Haridas V, Yaakob Z, K RN et al (2021) Selective electrochemical determination of paracetamol using hematite/graphene nanocomposite modified electrode prepared in a green chemical route. Mater Chem Phys 263:124379. https://doi.org/10.1016/j.matchemphys.2021.124379
Luo Y, Yang Y, Wang L et al (2022) An ultrafine ZnO/ZnNi2O4@porous carbon@covalent-organic framework for electrochemical detection of paracetamol and tert-butyl hydroquinone. J Alloys Compd 906:164369. https://doi.org/10.1016/j.jallcom.2022.164369
Kusuma KB, Manju M, Ravikumar CR et al (2022) Photocatalytic degradation of Methylene Blue and electrochemical sensing of paracetamol using Cerium oxide nanoparticles synthesized via sonochemical route. Appl Surf Sci Adv 11:100304. https://doi.org/10.1016/j.apsadv.2022.100304
Shalauddin M, Akhter S, Basirun WJ et al (2022) Carboxylated nanocellulose dispersed nitrogen doped graphene nanosheets and sodium dodecyl sulfate modified electrochemical sensor for the simultaneous determination of paracetamol and naproxen sodium. Measurement 194:110961. https://doi.org/10.1016/j.measurement.2022.110961
Leve ZD, Jahed N, Sanga NA et al (2022) Determination of Paracetamol on Electrochemically Reduced Graphene Oxide–Antimony Nanocomposite Modified Pencil Graphite Electrode Using Adsorptive Stripping Differential Pulse Voltammetry. Sensors 22:5784. https://doi.org/10.3390/s22155784
Farag AS (2022) Voltammetric determination of acetaminophen in pharmaceutical preparations and human urine using glassy carbon paste electrode modified with reduced graphene oxide. Anal Sci 38:1213–1220. https://doi.org/10.1007/s44211-022-00150-2
Han H, Liu C, Sha J et al (2021) Ferrocene-reduced graphene oxide-polyoxometalates based ternary nanocomposites as electrochemical detection for acetaminophen. Talanta 235:122751. https://doi.org/10.1016/j.talanta.2021.122751
Karuppusamy N, Mariyappan V, Chen SM et al (2023) Unveiling electrocatalytic performance of MnCo-P on sulfur-doped reduced graphene oxide for electrochemical detection of acetaminophen. Surf Interfaces 37:102681. https://doi.org/10.1016/j.surfin.2023.102681
Demir N, Atacan K, Ozmen M, Bas SZ (2020) Design of a new electrochemical sensing system based on MoS 2 –TiO 2 /reduced graphene oxide nanocomposite for the detection of paracetamol. New J Chem 44:11759–11767. https://doi.org/10.1039/D0NJ02298E
Chuenjitt S, Kongsuwan A, Phua CH et al (2022) A poly(neutral red)/porous graphene modified electrode for a voltammetric hydroquinone sensor. Electrochim Acta 434:141272. https://doi.org/10.1016/j.electacta.2022.141272
Wang C, Zhao P, Zhang L et al (2022) Switched electrochemical sensor for hydroquinone based on rGO@Au, monoclinic BiVO4 and temperature-sensitive polymer composite material. Microchem J 179:107412. https://doi.org/10.1016/j.microc.2022.107412
Al-Shekaili A, Al-Shukaili W, Khudaish EA (2022) A surface network based on oxidative graphene oxide for the determination of hydroquinone and catechol in ground and wastewater samples. J Electroanal Chem 919:116509. https://doi.org/10.1016/j.jelechem.2022.116509
Xia Y, Wang K, Shi Y et al (2021) Reduced graphene oxide cross-linked L-cysteine modified glassy carbon electrode for detection of environmental pollutant of hydroquinone. FlatChem 25:100214. https://doi.org/10.1016/j.flatc.2020.100214
Huang L, Cao Y, Diao D (2020) Electrochemical activation of graphene sheets embedded carbon films for high sensitivity simultaneous determination of hydroquinone, catechol and resorcinol. Sensors Actuators B Chem 305:127495. https://doi.org/10.1016/j.snb.2019.127495
Fan Z-C, Li Z, Wei X-Y et al (2022) Longquan lignite-derived hierarchical porous carbon electrochemical sensor for simultaneous detection of hazardous catechol and hydroquinone in environmental water samples. Microchem J 182:107880. https://doi.org/10.1016/j.microc.2022.107880
Park J, Kim J, Min A, Choi MY (2022) Fabrication of nonenzymatic electrochemical sensor based on Zn@ZnO core-shell structures obtained via pulsed laser ablation for selective determination of hydroquinone. Environ Res 204:112340. https://doi.org/10.1016/j.envres.2021.112340
Chetankumar K, Kumara Swamy BE, Sharma SC (2020) Electrochemical preparation of poly (direct yellow 11) modified pencil graphite electrode sensor for catechol and hydroquinone in presence of resorcinol: A voltammetric study. Microchem J 156:104979. https://doi.org/10.1016/j.microc.2020.104979
Jahani PM, Nejad FG, Dourandish Z et al (2022) A modified carbon paste electrode with N-rGO/CuO nanocomposite and ionic liquid for the efficient and cheap voltammetric sensing of hydroquinone in water specimens. Chemosphere 302:134712. https://doi.org/10.1016/j.chemosphere.2022.134712
Yi Y, Fiston MN, Zhang D, Zhu G (2020) Nitrogen-Doped Carbon Black/Reduced Graphene Oxide Nanohybrids for Simultaneous Electrochemical Determination of Hydroquinone and Paracetamol. J Electrochem Soc 167:066510. https://doi.org/10.1149/1945-7111/ab80cc
Meskher H, Achi F, Zouaoui A et al (2022) Simultaneous and Selective Electrochemical Determination of Catechol and Hydroquinone on A Nickel Oxide (NiO) Reduced Graphene Oxide (rGO) Doped Multiwalled Carbon Nanotube (fMWCNT) Modified Platinum Electrode. Anal Lett 55:1466–1481. https://doi.org/10.1080/00032719.2021.2008951
Chang F, Wang H, He S et al (2021) Simultaneous determination of hydroquinone and catechol by a reduced graphene oxide–polydopamine–carboxylated multi-walled carbon nanotube nanocomposite. RSC Adv 11:31950–31958. https://doi.org/10.1039/D1RA06032E
Rajeswari B, Sravani B, Cheffena M et al (2023) Ethylene glycol-assisted synthesis of reduced graphene oxide-supported bimetallic Pt-Co nanoparticles for the ultra-sensitive detection of tert-butyl hydroquinone. Inorg Chem Commun 151:110627. https://doi.org/10.1016/j.inoche.2023.110627
Liao L, Zhou P, Xiao F et al (2023) Electrochemical sensor based on Ni/N-doped graphene oxide for the determination of hydroquinone and catechol. Ionics (Kiel) 29:1605–1615. https://doi.org/10.1007/s11581-023-04892-5
Rocha DP, Dornellas RM, Cardoso RM et al (2018) Chemically versus electrochemically reduced graphene oxide: Improved amperometric and voltammetric sensors of phenolic compounds on higher roughness surfaces. Sensors Actuators B Chem 254:701–708. https://doi.org/10.1016/j.snb.2017.07.070
Acknowledgements
The authors are grateful to UFV/DEQ for the infrastructure.
Funding
This work received financial support from CAPES, CNPq, and FAPEMIG (Grant Numbers: RED-00144-22, APQ-0008321, and APQ-03113-22).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 464 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silva, R.M., Sperandio, G.H., da Silva, A.D. et al. Electrochemically reduced graphene oxide films from Zn-C battery waste for the electrochemical determination of paracetamol and hydroquinone. Microchim Acta 190, 273 (2023). https://doi.org/10.1007/s00604-023-05858-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05858-0