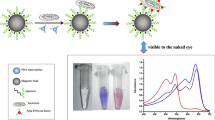
Abstract
An innovative aptamer labeled with 5-FAM has been developed with a high affinity for Yersinia enterocolitica (Y. enterocolitica) using graphene oxide (GO) as a quenching platform. The selectivity of the prepared system was evaluated in the presence of common coexisted bacteria like Yersinia pseudotuberculosis, Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, and Salmonella typhimurium. Some experimental factors like pH and stability were investigated. The results showed that in the absence of Y. enterocolitica, aptamer labeled with 5-FAM was bonded with GO, causing fluorescence to be relatively weak. After the addition of Y. enterocolitica, the aptamer is released from the GO surface and binds to the target bacteria, and significantly increases the fluorescence intensity with an excitation wavelength of 410 nm and an emission wavelength of 530 nm. After optimizing all conditions, the system exhibited a wide linear response for Y. enterocolitica in the concentration range 10 to 1.0 × 109 CFU•mL–1 and the limit of detection (LOD) was 3 CFU•mL–1. This system demonstrated that GO-designed aptamers can be successful in detecting Y. enterocolitica in whole-cell forms, making them potentially useful for screening and rapid detection.
Graphical Abstract







Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author, [Ali Ehsani], upon reasonable request.
References
Authority EFS (2017) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2016. EFSA journal 15 (12)
Authority EFS, Prevention ECfD, Control (2018) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J 16(12):e05500
CDC CfDCaP (2016) CDC, Centers for Disease Control and Prevention. Available at https://www.cdcgov/yersinia/(2016). (updated October 24, 2016)
de Boer E, Beumer RR (1999) Methodology for detection and typing of foodborne microorganisms. Int J Food Microbiol 50(1–2):119–130
Arora P, Sindhu A, Dilbaghi N, Chaudhury A (2011) Biosensors as innovative tools for the detection of food borne pathogens. Biosens Bioelectron 28(1):1–12
Peruzy M, Aponte M, Proroga Y, Capuano F, Cristiano D, Delibato E, Houf K, Murru N (2020) Yersinia enterocolitica detection in pork products: evaluation of isolation protocols. Food Microbiol 92:103593
Thevenot DR, Toth K, Durst RA, Wilson GS (1999) Electrochemical biosensors: recommended definitions and classification. Pure Appl Chem 71(12):2333–2348
Tombelli S, Minunni M, Mascini M (2007) Aptamers-based assays for diagnostics, environmental and food analysis. Biomol Eng 24(2):191–200
Shoaib M, Shehzad A, Mukama O, Raza H, Niazi S, Khan IM, Ali B, Akhtar W, Wang Z (2020) Selection of potential aptamers for specific growth stage detection of Yersinia enterocolitica. RSC Adv 10(41):24743–24752
Liu M, Yue F, Kong Q, Liu Z, Guo Y, Sun X (2022) Aptamers against pathogenic bacteria: selection strategies and apta-assay/aptasensor application for food safety. J Agric Food Chem 70(18):5477–5498
Babaei A, Zendehdel M, Khalilzadeh B, Abnosi M (2010) A new sensor for simultaneous determination of tyrosine and dopamine using iron (III) doped zeolite modified carbon paste electrode. Chin J Chem 28(10):1967–1972
Labuda J, Brett AMO, Evtugyn G, Fojta M, Mascini M, Ozsoz M, Palchetti I, Paleček E, Wang J (2010) Electrochemical nucleic acid-based biosensors: Concepts, terms, and methodology (IUPAC Technical Report). Pure Appl Chem 82(5):1161–1187
Subjakova V, Oravczova V, Tatarko M, Hianik T (2021) Advances in electrochemical aptasensors and immunosensors for detection of bacterial pathogens in food. Electrochim Acta 389:138724
Mansouri M, Khalilzadeh B, Barzegari A, Shoeibi S, Isildak S, Bargahi N, Omidi Y, Dastmalchi S, Rashidi M-R (2020) Design a highly specific sequence for electrochemical evaluation of meat adulteration in cooked sausages. Biosens Bioelectron 150:111916
Majdinasab M, Hayat A, Marty JL (2018) Aptamer-based assays and aptasensors for detection of pathogenic bacteria in food samples. TrAC Trends Anal Chem 107:60–77. https://doi.org/10.1016/j.trac.2018.07.016
Nasrollahpour H, Mahdipour M, Isildak I, Rashidi M-R, Naseri A, Khalilzadeh B (2021) A highly sensitive electrochemiluminescence cytosensor for detection of SKBR-3 cells as metastatic breast cancer cell line: a constructive phase in early and precise diagnosis. Biosens Bioelectron 178:113023
Ping J, Zhou Y, Wu Y, Papper V, Boujday S, Marks RS, Steele TW (2015) Recent advances in aptasensors based on graphene and graphene-like nanomaterials. Biosens Bioelectron 64:373–385
Isildak I, Navaeipour F, Afsharan H, Kanberoglu GS, Agir I, Ozer T, Annabi N, Totu EE, Khalilzadeh B (2020) Electrochemiluminescence methods using CdS quantum dots in aptamer-based thrombin biosensors: a comparative study. Microchim Acta 187:1–13
Nasrollahpour H, Isildak I, Rashidi M-R, Hashemi EA, Naseri A, Khalilzadeh B (2021) Ultrasensitive bioassaying of HER-2 protein for diagnosis of breast cancer using reduced graphene oxide/chitosan as nanobiocompatible platform. Cancer Nanotechnol 12(1):10
Jin B, Wang S, Lin M, Jin Y, Zhang S, Cui X, Gong Y, Li A, Xu F, Lu TJ (2017) Upconversion nanoparticles based FRET aptasensor for rapid and ultrasenstive bacteria detection. Biosens Bioelectron 90:525–533
Chenaghlou S, Khataee A, Jalili R, Rashidi M-R, Khalilzadeh B, Joo SW (2021) Gold nanostar-enhanced electrochemiluminescence immunosensor for highly sensitive detection of cancer stem cells using CD133 membrane biomarker. Bioelectrochem 137:107633
Ocsoy MA, Yusufbeyoglu S, Ildiz N, Ulgen A, Ocsoy I (2021) DNA aptamer-conjugated magnetic graphene oxide for pathogenic bacteria aggregation: selective and enhanced photothermal therapy for effective and rapid killing. ACS Omega 6(31):20637–20643. https://doi.org/10.1021/acsomega.1c02832
Wu G, Dai Z, Tang X, Lin Z, Lo PK, Meyyappan M, Lai KWC (2017) Graphene field-effect transistors for the sensitive and selective detection of Escherichia coli using pyrene-tagged DNA aptamer. Adv Healthc Mater 6(19). https://doi.org/10.1002/adhm.201700736
Duan YF, Ning Y, Song Y, Deng L (2014) Fluorescent aptasensor for the determination of Salmonella typhimurium based on a graphene oxide platform. Microchim Acta 181(5–6):647–653. https://doi.org/10.1007/s00604-014-1170-4
Ling M, Peng Z, Cheng L, Deng L (2015) Rapid fluorescent detection of enterotoxigenic Escherichia coli (ETEC) K88 based on graphene oxide-dependent nanoquencher and Klenow fragment-triggered target cyclic amplification. Appl Spectrosc 69(10):1175–1181. https://doi.org/10.1366/15-07881
Gao R, Zhong Z, Gao X, Jia L (2018) Graphene oxide quantum dots assisted construction of fluorescent aptasensor for rapid detection of pseudomonas aeruginosa in food samples. J Agric Food Chem 66(41):10898–10905. https://doi.org/10.1021/acs.jafc.8b02164
Zhu W, Li Z, Liu X, Yan X, Deng L (2015) Determination of Shigella flexneri by a novel fluorescent aptasensor. Anal Lett 48(18):2870–2881. https://doi.org/10.1080/00032719.2015.1052974
Bayraç AT, Donmez SI (2018) Selection of DNA aptamers to Streptococcus pneumonia and fabrication of graphene oxide based fluorescent assay. Anal Biochem 556:91–98. https://doi.org/10.1016/j.ab.2018.06.024
Appaturi JN, Pulingam T, Thong KL, Muniandy S, Ahmad N, Leo BF (2020) Rapid and sensitive detection of Salmonella with reduced graphene oxide-carbon nanotube based electrochemical aptasensor. Anal Biochem 589:113489. https://doi.org/10.1016/j.ab.2019.113489
Appaturi JN, Pulingam T, Thong KL, Muniandy S, Ahmad N, Leo BF (2020) Rapid and sensitive detection of Salmonella with reduced graphene oxide-carbon nanotube based electrochemical aptasensor. Anal Biochem 589. https://doi.org/10.1016/j.ab.2019.113489
Muniandy S, Teh SJ, Appaturi JN, Thong KL, Lai CW, Ibrahim F, Leo BF (2019) A reduced graphene oxide-titanium dioxide nanocomposite based electrochemical aptasensor for rapid and sensitive detection of Salmonella enterica. Bioelectrochem 127:136–144. https://doi.org/10.1016/j.bioelechem.2019.02.005
Chinnappan R, AlAmer S, Eissa S, Rahamn AA, Abu Salah KM, Zourob M (2018) Fluorometric graphene oxide-based detection of Salmonella enteritis using a truncated DNA aptamer. Microchim Acta 185(1):1–9
Wang H, Chi Z, Cong Y, Wang Z, Jiang F, Geng J, Zhang P, Ju P, Dong Q, Liu C (2018) Development of a fluorescence assay for highly sensitive detection of: pseudomonas aeruginosa based on an aptamer-carbon dots/graphene oxide system. RSC Adv 8(57):32454–32460. https://doi.org/10.1039/c8ra04819c
Chinnappan R, AlAmer S, Eissa S, Rahamn AA, Abu Salah KM, Zourob M (2017) Fluorometric graphene oxide-based detection of Salmonella enteritis using a truncated DNA aptamer. Microchim Acta 185(1):61. https://doi.org/10.1007/s00604-017-2601-9
Sheikhzadeh E, Chamsaz M, Turner APF, Jager EWH, Beni V (2016) Label-free impedimetric biosensor for Salmonella Typhimurium detection based on poly [pyrrole-co-3-carboxyl-pyrrole] copolymer supported aptamer. Biosens Bioelectron 80:194–200. https://doi.org/10.1016/j.bios.2016.01.057
Zhao X, Cui Y, Wang J, Wang J (2019) Preparation of fluorescent molecularly imprinted polymers via pickering emulsion interfaces and the application for visual sensing analysis of Listeria Monocytogenes. Polymers 11(6):984
Yin M, Wu C, Li H, Jia Z, Deng Q, Wang S, Zhang Y (2019) Simultaneous sensing of seven pathogenic bacteria by guanidine-functionalized upconversion fluorescent nanoparticles. ACS Omega 4(5):8953–8959. https://doi.org/10.1021/acsomega.9b00775
Jia F, Duan N, Wu S, Dai R, Wang Z, Li X (2016) Impedimetric Salmonella aptasensor using a glassy carbon electrode modified with an electrodeposited composite consisting of reduced graphene oxide and carbon nanotubes. Microchim Acta 183(1):337–344. https://doi.org/10.1007/s00604-015-1649-7
Alhogail S, Suaifan GARY, Zourob M (2016) Rapid colorimetric sensing platform for the detection of Listeria monocytogenes foodborne pathogen. Biosens Bioelectron 86:1061–1066. https://doi.org/10.1016/j.bios.2016.07.043
Funding
The research protocol was approved and supported by the Student Research Committee, Tabriz University of Medical Sciences (grant number: 68658). Also, this study has been approved and supported by the Department of Food Science and Technology, Faculty of Nutrition and Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran. We gratefully acknowledge their assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Milad Tavassoli and Arezou Khezerlou are equal first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tavassoli, M., Khezerlou, A., Hamishehkar, H. et al. An ultrasensitive aptamer-based fluorescent on/off system for trace amount evaluation of Yersinia enterocolitica in food samples. Microchim Acta 190, 253 (2023). https://doi.org/10.1007/s00604-023-05820-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05820-0