Abstract
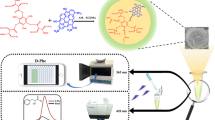
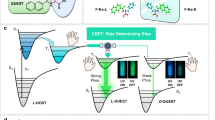
A supersensitive chiroptical-responsive system of enantioselectively recognizing L- and D-tryptophan (Trp) based on ( +)-diacetyl-L-tartaric anhydride-functionalized 1,3,5-triformylphloroglucinol (DTA-functionalized Tp) was constructed for the first time. With a high fluorescence quantum yield of 15.2% and fluorescence lifetime of 57.6 μs, DTA-functionalized Tp as both fluorescent and chiral recognition nanoprobe was used for the discrimination of L- and D-Trp with excitation/emission maxima at 330/490 nm within 3 min. The linear range of the fluorescence sensing was 0.002–0.15 μg mL−1, and the detection limit achieved 1.4 ng mL−1. Furthermore, a smartphone was employed as a detector and processor to couple with the chiroptical-responsive nanoprobe for establishing a novel and visual integration system for rapid and real-time detection of chiral amino acids with a detection limit of 13 ng mL−1. The spiked recoveries of L-Trp in two commercially available functional beverages ranged from 86.00 to 118.33% in fluorescence and smartphone-based sensing system. Based on the excellent chiroptical-responsive effects, high stability, and biocompatibility, the chiroptical-responsive nanoprobe was successfully applied to visual optosensing and fluorescence imaging in response to L- and D-Trp in HeLa cells. This discrimination methodology with high sensitivity and enantioselectively shows great potential for in-site visually monitoring chiral amino acids in real food samples and tracking physiological processes.
Graphical abstract









Similar content being viewed by others
References
Hu X, Guo F (2020) Amino acid sensing in metabolic homeostasis and health. Endocr Rev 42(1):56–76. https://doi.org/10.1016/j.fshw.2015.01.001
Liang RP, Liu CM, Meng XY, Wang JW, Qiu JD (2012) A novel open-tubular capillary electrochromatography using β-cyclodextrin functionalized graphene oxide-magnetic nanocomposites as tunable stationary phase. J Chromatogr A 1266:95–102. https://doi.org/10.1016/j.chroma.2012.09.101
Waldhier MC, Almstetter MF, Nürnberger N, Gruber MA, Dettmer K, Oefner PJ (2011) Improved enantiomer resolution and quantification of free D-amino acids in serum and urine by comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. J Chromatogr A 1218:4537–4544. https://doi.org/10.1016/j.chroma.2011.05.039
Zhang Z, Liu Y, Liu P, Yang L, Jiang X, Luo D, Yang D (2017) Non-invasive detection of gastric cancer relevant d-amino acids with luminescent DNA/silver nanoclusters. Nanoscale 9:19367–19373. https://doi.org/10.1039/c7nr07337b
Niu X, Yang X, Mo Z, Guo R, Liu N, Zhao P, Ouyang M (2019) Voltammetric enantiomeric differentiation of tryptophan by using multiwalled carbon nanotubes functionalized with ferrocene and β-cyclodextrin. Electrochim Acta 297:650–659. https://doi.org/10.1016/j.electacta.2018.12.041
Song J, Yang C, Ma J, Han Q, Ran P, Fu Y (2018) Voltammetric chiral discrimination of tryptophan using a multilayer nanocomposite with implemented amino-modified β-cyclodextrin as recognition element. Microchim Acta 185:230. https://doi.org/10.1007/s00604-018-2765-y
Sardella R, Lisanti A, Marinozzi M, Ianni F, Natalini B, Blanch GP, Castillob ML (2013) Combined monodimensional chromatographic approaches to monitor the presence of D-amino acids in cheese. Food Control 34:478–487. https://doi.org/10.1016/j.foodcont.2013.05.026
Rocco A, Aturki Z, Fanali S (2013) Chiral separations in food analysis. TrAC Trends Anal Chem 52:206–225. https://doi.org/10.1016/j.trac.2017.05.013
Booth TD, Wahnon D, Wainer IW (1997) Is chiral recognition a three-point process? Chirality 9:96–98. https://doi.org/10.1002/(SICI)1520-636X(1997)9:2%3c96::AID-CHIR2%3e3.0.CO;2-E
Teixeira J, Tiritan ME, Pinto MMM, Fernandes C (2019) Chiral stationary phases for liquid chromatography: recent developments. Molecules 24:865. https://doi.org/10.3390/molecules24050865
Jágerszki G, Takács A, Bitter I, Gyurcsányi RE (2011) Solid-state ion channels for potentiometric sensing. Angew Chem Int Ed 50:1656–1659. https://doi.org/10.1002/anie.201003849
Zhang J, Albelda MT, Liu Y, Canary JW (2005) Chiral nanotechnology. Chirality 17:404–420. https://doi.org/10.1002/chir.20178
Huang J, Egan VM, Guo H, Yoon JY, Briseno AL, Rauda IE, Garrell RL, Knobler CM, Zhou F, Kaner RB (2003) Enantioselective discrimination of D- and L-phenylalanine by chiral polyaniline thin films. Adv Mater 15:1158–1161. https://doi.org/10.1002/adma.200304835
Zhang L, Xu C, Liu C, Li B (2014) Visual chiral recognition of tryptophan enantiomers using unmodified gold nanoparticles as colorimetric probes. Anal Chim Acta 809:123–127. https://doi.org/10.1016/j.aca.2013.11.043
Duan C, Won M, Verwilst P, Xu J, Kim HS, Zeng L, Kim JS (2019) In vivo imaging of endogenously produced HClO in zebrafish and mice using a bright, photostable ratiometric fluorescent probe. Anal Chem 91:4172–4178. https://doi.org/10.1021/acs.analchem.9b00224
Huang H, Chen B, Li L, Wang Y, Shen Z, Wang Y, Li X (2022) A two-photon fluorescence probe with endoplasmic reticulum targeting ability for turn-on sensing photosensitized singlet oxygen in living cells and brain tissues. Talanta 237:122963. https://doi.org/10.1016/j.talanta.2021.122963
Zhu X, Han L, Liu H, Sun B (2022) A smartphone-based ratiometric fluorescent sensing system for on-site detection of pyrethroids by using blue-green dual-emission carbon dots. Food Chem 379:132154. https://doi.org/10.1016/j.foodchem.2022.132154
Kabe R, Adachi C (2017) Organic long persistent luminescence. Nature 550:384–387. https://doi.org/10.1038/nature24010
Kwak SY, Giraldo JP, Wong MH, Koman VB, Lew TTS, Ell J, Weidman MC, Sinclair RM, Landry MP, Tisdale WA, Strano MS (2017) A nanobionic light-emitting plant. Nano Lett 17:7951–7961. https://doi.org/10.1021/acs.nanolett.7b04369
Li S, Su W, Wu H, Yuan T, Yuan C, Liu J, Deng G, Gao X, Chen Z, Bao Y, Yuan F, Zhou S, Tan H, Li Y, Li X, Fan L, Zhu J, Chen AT, Liu F, Zhou Y, Li M, Zhai X, Zhou J (2020) Targeted tumour theranostics in mice via carbon quantum dots structurally mimicking large amino acids. Nat Biomed Eng 4:704–716. https://doi.org/10.1038/s41551-020-0540-y
Liu JM, Hu Y, Yang YK, Liu H, Fang GZ, Lu X, Wang S (2018) Emerging functional nanomaterials for the detection of food contaminants. Trends Food Sci Tech 71:94–106. https://doi.org/10.1016/j.tifs.2017.11.005
Xu X, An H, Zhang D, Tao H, Dou Y, Li X, Huang J, Zhang J (2019) A self-illuminating nanoparticle for inflammation imaging and cancer therapy. Sci Adv 5:eaat2953. https://doi.org/10.1126/sciadv.aat2953
Clough JM, Balan A, van Daal TLJ, Sijbesma RP (2016) Probing force with mechanobase-induced chemiluminescence. Angew Chem Int Ed 55:1445–1449. https://doi.org/10.1002/anie.201508840
Gnaim S, Shabat D (2017) Self-immolative chemiluminescence polymers: innate assimilation of chemiexcitation in a domino-like depolymerization. J Am Chem Soc 139:10002–10008. https://doi.org/10.1021/jacs.7b04804
Gnaim S, Scomparin A, Das S, Blau R, Satchi-Fainaro R, Shabat D (2018) Direct real-time monitoring of prodrug activation by chemiluminescence. Angew Chem Int Ed 57:9033–9037. https://doi.org/10.1002/anie.201804816
Hananya N, Shabat D (2019) Recent advances and challenges in luminescent imaging: bright outlook for chemiluminescence of dioxetanes in water. ACS Central Sci 5:949–959. https://doi.org/10.1021/acscentsci.9b00372
Xiao X, Wu T, Cao J, Zhu C, Liu Y, Zhang X, Shen Y (2020) Rational engineering of chromic material as near-infrared ratiometric fluorescent nanosensor for H2S monitoring in real food samples. Sens Actuators B 323:128707. https://doi.org/10.1016/j.snb.2020.128707
Yuan X, Jiang W, Wang J, Liu H, Sun B (2020) High-performance multiporous imprinted microspheres based on N-doped carbon dots exfoliated from covalent organic framework for flonicamid optosensing. ACS Appl Mater Inter 12:25150–25158. https://doi.org/10.1021/acsami.0c04766
Yuan X, Zhang D, Zhu X, Liu H, Sun B (2021) Triple-dimensional spectroscopy combined with chemometrics for the discrimination of pesticide residues based on ionic liquid-stabilized Mn-ZnS quantum dots and covalent organic frameworks. Food Chem 342:128299. https://doi.org/10.1016/j.foodchem.2020.128299
Zhu X, Jiang W, Zhao Y, Liu H, Sun B (2021) Single, dual and multi-emission carbon dots based optosensing for food safety. Trends Food Sci Tech 111:388–404. https://doi.org/10.1016/j.tifs.2021.03.005
Zhu X, Yuan X, Han L, Liu H, Sun B (2021) A smartphone-integrated optosensing platform based on red-emission carbon dots for real-time detection of pyrethroids. Biosens Bioelectron 191:113460. https://doi.org/10.1016/j.bios.2021.113460
Kandambeth S, Mallick A, Lukose B, Mane MV, Heine T, Banerjee R (2012) Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J Am Chem Soc 134:19524–19527. https://doi.org/10.1021/ja308278w
Qian HL, Yang CX, Yan XP (2016) Bottom-up synthesis of chiral covalent organic frameworks and their bound capillaries for chiral separation. Nat Commun 7:12104. https://doi.org/10.1038/ncomms12104
Kou WT, Yang CX, Yan XP (2018) Post-synthetic modification of metal–organic frameworks for chiral gas chromatography. J Mater Chem A 6:17861–17866. https://doi.org/10.1039/C8TA06804F
Armstrong DW, Ward TJ, Armstrong RD, Beesley TE (1986) Separation of drug stereoisomers by the formation of β-cyclodextrin inclusion complexes. Science 232(4754):1132–1135. https://doi.org/10.1126/science.3704640
Zhao Y, Liu H, Sun B (2022) Chiral induction in carbazole-conjugated covalent organic frameworks: a supersensitive fluorescence sensing platform for chiral recognition. Sensor Actuat B: Chem 354:131253. https://doi.org/10.1016/j.snb.2021.131253
Luo X, Han Y, Chen X, Tang W, Yue T, Li Z (2020) Carbon dots derived fluorescent nanosensors as versatile tools for food quality and safety assessment: a review. Trends Food Sci Tech 95:149–161. https://doi.org/10.1016/j.tifs.2019.11.017
Zhu S, Song Y, Zhao X, Shao J, Zhang J, Yang B (2015) The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Res 8:355–381. https://doi.org/10.1007/s12274-014-0644-3
Funding
This work was supported by the National Natural Science Foundation of China (No. 31822040, 32072335) and the National Key R&D Program of China (No. 2018YFC1602300).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing financial interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, Y., Yuan, X., Jiang, W. et al. Chiroptical-responsive nanoprobe for the optosensing of chiral amino acids. Microchim Acta 189, 184 (2022). https://doi.org/10.1007/s00604-022-05282-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05282-w