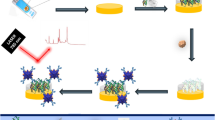
Abstract
Molecularly imprinted polymers MIPs were successfully assembled around quantum dots (QDs), for the detection of the protein biomarker CA19-9 associated to pancreatic cancer (PC). These imprinted materials MIP@QDs were incorporated within the cellulose hydrogel with retention of its conformational structure inside the binding cavities. The concept is to use MIPs which function as the biorecognition elements, conjugated to cadmium telluride QDs as the sensing system. The excitation wavelength was set to 477 nm and the fluorescence signal was measured at its maximum intensity, with an emission range between 530 and 780 nm. The fluorescence quenching of the imprinted cellulose hydrogels occurred with increasing concentrations of CA19-9, showing linearity in the range 2.76 × 10 −2 – 5.23 × 10 2 U/ml, in a 1000-fold diluted human serum. Replicates of the imprinted hydrogel show a linear response below the cut-off values for pancreatic cancer diagnosis (< 23 U/ml), a limit of detection of 1.58 × 10 −3 U/ml and an imprinting factor (IF) of 1.76. In addition to the fact that the imprinted cellulose hydrogel displays good stability and selectivity towards CA19-9 when compared with the non-imprinted controls, the conjugation of MIPs to QDs increases the sensitivity of the system for an optical detection method towards ranges within clinical significance. This fact shows potential for the imprinted hydrogel to be applied as a sensitive, low-cost format for point-of-care tests (PoCTs).
Graphical abstract

Similar content being viewed by others
Introduction
Pancreatic cancer (PC) remains a leading cause of cancer-related deaths, accounting for more than 7% of cancer deaths among men and women [1]. Carbohydrate antigen 19–9 (CA 19–9) is a glycosylated mucin-type protein biomarker with an average molecular weight of 210 kDa, that is released by pancreatic tumour cells. Its serum concentration is low in healthy individuals (< 37 U/ml), in contrast to higher values in cancer patients [2]. Ca19-9 monitoring is especially utilized after PC surgical treatment, for surveillance of the disease [3, 4]. PC recurrence is usually diagnosed and monitored using advanced imageology techniques, such as computerized tomography scanning, endoscopy, and magnetic resonance imaging [5]. Other traditional methods include immunoassays based on electro-chemiluminescent methods [6, 7] and radio-immunoassays [8]. Although they present high sensitivity and prevail as the golden clinical standards, they are increasingly costly. Moreover, they are usually associated with elaborated protocols, requiring the use of expensive instrumentations and specialized personnel. Its main prejudice is the prolonged time needed to provide the results. Nowadays, the pursuit of rapid and accessible tests has prompted the development of new PoCTs in the context of a user-friendly diagnosis. Although most of these tests are immunoassays, some drawbacks, such as instability over time, may cause some inaccurate results. Despite this, new studies have been developed using PoCTs, either electrochemically [9,10,11], either using fluorescence spectroscopy [12,13,14] or surface plasmon resonance [15]. To overcome some disadvantages of the immunoassays, MIPs arise as biomimetic sensors of a target analyte, functioning as the biorecognition elements of that target [16, 17]. They provide the stability of the sensor, namely their resistance to environmental changes and high physicochemical stability, e.g., in various solvents, pHs or temperatures, while privileging selectivity for the analyte imprinted [18, 19]. The free radical polymerization strategy is a common polymerization method that possesses high tolerance for a wide range of functional monomers and great adaptability to all kinds of reaction conditions. Even so, its largest drawback is that it should be spatially controlled and functionally adaptable, both in order to avoid overgrowth of the polymer around the template, as well as to maintain the binding affinity provided by the conformational cavity around the target molecule [20]. Even though, the application of MIPs in proteins and other macromolecules still faces major challenges because of the greater mass transfer resistance during the imprinting of these large molecules as compared with small molecules [16]. Despite the use of QDs to detect CA19-9 in immunoassays being documented [21,22,23], to the best of our knowledge, the conjugation of MIPs with QDs for CA19-9 detection has scarcely been reported [13, 24].
In this article, MIPs were assembled at the surface of fluorescent red-emitting CdTe@MPA QDs in aqueous media and optimized for the specific detection of the protein biomarker CA19-9 in human serum. These nanoparticles herein designated as red QDs or simply QDs, are semiconducting nanocrystals that were used as the optical sensing probes for the target analyte. Surface imprinting was the polymerization strategy employed, which enables more precise control of the polymer growth around the template molecule than classic bulk polymerization [25,26,27,28,29,30]. The imprinted cellulose hydrogels were compared to its non-imprinted controls and evaluated towards its potential to be applied as a user-friendly format system for PoCTs. Cellulose hydrogel is a biocompatible and biodegradable natural polymer that was used as the physical support to embed the biorecognition elements MIP@QDs. It has been reported that a hydrogel coating around inorganic and metal nanoparticles confers a hydration layer, which often is an essential prerequisite for biomedical and biological applications [31]. This hydration layer can significantly improve the biocompatibility and reduce the cytotoxicity of the QDs nanoparticles as reported by other authors. However, parameters such as concentration and uniform distribution of the nanoparticles need to be balanced, to prevent its agglomeration [27, 32]. Overall, a sensitive imprinted hydrogel HEC:MIP@QDs was developed for the detection of the CA19-9 protein biomarker using cellulose as biocompatible support.
Experimental section
Information related to reagents and materials, instrumentation and optical measurements can be found in the Electronic Supplementary Information S1. EXPERIMENTAL SECTION.
Synthesis of the imprinted materials
Red-emitting cadmium telluride quantum dots capped with 3-mercaptopropionic acid, CdTe@MPA QDs, were prepared according to our previous work [33]. The imprinted polymers MIP@QDs and the correspondent non-imprinted controls NIP@QDs, herein both designated as conjugates CJG@QDs, were prepared by the surface imprinting strategy using the free radical polymerization method, as shown in Fig. 1. Briefly, 1kU of CA19-9 protein was incubated with 1 ml of red emitting QDs 5.36 × 10–6 M in PBS 10 mM pH 6.0, during 3hs room temperature (r.t.). Next, AEMH (8.34 × 10−6 M) was added and the reaction mixture and stirred for 2 hs (r.t.). The solution was centrifuged, and the supernatants were discarded. The reaction pellet was resuspended in 1 ml PBS 10 mM pH 6.0 and the functional monomer 2-hydroxyethyl methacrylate (HEMA) (8.20 × 10–5 M), and the crosslinker trimethylpropane trimethacrylate (TMPTMA) (5.30 × 10–5 M) were added. The reaction mixture was degassed with N2 for 2 min., then tetramethylethylenediamine (TEMED) (7.03 × 10–6 M) and ammonium persulfate (APS) (8.12 × 10–6 M) were added to initiate the polymerization and the reaction mixture was stirred for 1 h at r.t. During the washings, both CJG@QDs were centrifuged and resuspended in (carbonate/bicarbonate) buffer 10 mM pH 9.2 for several cycles, until no protein was detected in the supernatants of the MIPCA19-9@QDs by UV–vis spectroscopy (Figure S1A_3 and S1B_b and c). The synthesis of NIP@QDs followed the same strategy but without the protein incubation (Figure S1A_5 and 6). Upon washings, both conjugates CJG@QDs were dried at 60ºC and kept in a desiccator protected from light. From here on, the washed imprinted polymers, from which the protein is extracted from the imprinted cavities, will be designated as MIP@QDs, to be distinguished from its polymerized state as MIPsCA19-9@QDs, which still contain the protein. The corresponding non-imprinted controls, as they do not hold the protein in its polymerized state, are always designated as NIP@QDs. The protocol was repeated in the preparation of MIPCA19-9@QDs, using 5kU of CA19-9 in the imprinting step.
Synthesis of molecularly imprinted polymers MIP@QDs for the detection of the protein CA19-9. Incubation of the protein (1); Adsorption of aminoethyl methacrylate hydrochloride (AEMH) (2); polymerization at the surface of QDs nanoparticles (3); removal of the protein from MIP@QDs (4); rebinding of the protein to the imprinted cellulose hydrogels HEC:MIP@QDs (5). Widefield deconvolution microscopy images of HEC:MIP@QDs in the presence of CA19-9 (A); in the absence of CA19-9 (B)
Synthesis of the cellulose hydrogels
The protocol followed our previous work, with small modifications [34]. Briefly, 250 mg HEC, 50 mg PVOH and 125 ml PEG were added to a glass beaker with 10 ml ultrapure water. The mixture was sonicated using 20% of vibration amplitude from the maximum value allowed by the instrument until it became a transparent hydrogel. The resulting hydrogel was kept at 4ºC.
until use. Details on the experiment can be found in the ESM file SECTION S2. RESULTS AND DISCUSSION.
Assembly of the imprinted materials
Both imprinted MIP@QDs and non-imprinted materials NIP@QDs prepared in Sect. 2.1 were dried in a desiccator and kept at r.t. protected from light. Then 20 mg of each material was resuspended in 1 ml of the hydrogel from Sect. 2.2 and the mixture was sonicated to obtain the correspondent imprinted hydrogels HEC:MIP@QDs (Figure S1_(A)_curve 4) and non-imprinted hydrogels HEC:NIP@QDs (Figure S1_(A)_curve 7). During sonication, and to guarantee that the imprinted materials were fully dispersed within the cellulose hydrogel, we studied the effect of ultrasonication duration, power, and pulse mode on de-agglomeration of QDs nanoparticles. Details on the experiment can be found in the ESM file SECTION S2. RESULTS AND DISCUSSION.
Results and discussion
Optimizations of the imprinted cellulose hydrogels
In this work, free radical polymerization was used as the imprinting approach at the surface of the QDs nanoparticles suspended in PBS, with control of the pH and time of polymerization. During optimization of the MIP@QDs, the surface imprinting strategy was preferred over bulk imprinting, having into consideration our previous works [25, 33]. In addition, it minimizes the overgrowth of the polymeric layer and avoids the entrapment of the biomolecule inside the polymer network [26,27,28,29]. Two imprinting concentrations of CA19-9 were tested for the assembly of the MIP@QDs (1kU/ml and 5kU/ml) (Fig. 7B, rose and yellow bars). Upon polymerization, the decrease in the fluorescence intensity of the MIPCA19-9@QDs (Fig. 7B, rose and yellow bars) is higher, relative to the non-imprinted controls NIP@QDs (Fig. 7B, green bars). This result may be attributed to the presence of the target molecule CA19-9 protein, which possesses a high molecular weight (~ 210 kDa). The alterations of the fluorescence intensity of the MIP@QDs upon imprinting 5kU/ml (Fig. 7B, yellow bar) is negligible relative to the imprinting of 1kU/ml of CA19-9 (Fig. 7B, rose bar) and for this reason it was decided to proceed with the lowest imprinting concentration. This experimental result may be explained by the fact that when using a higher concentration of large biomolecules, maintaining the other assembly conditions, it is reasonable to speculate that the large biomolecules may become aggregated. This in turn may compromise the structure of the binding cavity, which may become mismatched from the conformational structure of the target analyte. In addition, the excess of imprinting concentration may induce parts, or the entire protein structure to become entrapped within the polymer and difficult its removal. Two different pHs were tested. during the assembly of MIP@QDs. It was observed a more pronounced decrease in its fluorescence signal in PBS 10 mM pH 6.0 (Fig. 7B, rose bars) compared to its assembly in PBS 10 mM pH 7.2 (Fig. 7B, yellow bars). This result can be explained by the change in the zeta potential value of CA19-9 suspended in PBS at different pHs. The surface potential of the protein changed from (+ 19.53 ± 1.02) mV in PBS 10 mM pH 7.2 to (+ 25.76 ± 3.80) in PBS 10 mM pH 6.0, thus favouring the electrostatic interactions with the negatively charged QDs nanoparticles, in accordance with our previous results [33]. For this reason, we proceed with the calibrations in PBS 10 mM pH 6.0. Another optimization was the selection of the crosslinker. Two crosslinkers were chosen, ethylene glycol dimethacrylate (EGDMA), and trimethylpropane trimethacrylate (TMPTMA), using 1 h or 2hs polymerization (Fig. 7C). Prolonging the polymerization time to two hours did not benefit the imprinting process, as the fluorescence intensity of the washed MIP@QDs upon 2hs polymerization was lower compared with 1 h polymerization (Fig. 7C, dashed pink bars). In addition, EGDMA was revealed not to be a good alternative for the polymerization process. The washed MIP@QDs with EGDMA did not increase as much its fluorescence signal compared to MIP@QDs with TMPTMA (Fig. 7C, dashed pink bars). One possible explanation is that the polymeric network formed with the linear crosslinker is probably tighter as compared to the network formed with the branched crosslinker, thus enabling better permeation to the protein molecules through the imprinted MIP@QDs and more efficient protein removal from the polymeric matrix. Regarding the washing step to remove the protein from the imprinted MIP@QDs, it was found that enhancing the pH of the washing buffer facilitated the process of protein removal. The washed MIP@QDs increased its fluorescence signal (Fig. 7C, red line), compared to the non-imprinted NIP@QDs (Fig. 7C, blue line). These findings are in good agreement with the absence of protein observed in the UV/Vis spectra of the supernatants of MIP@QDs. The non-imprinted NIP@QDs maintained its fluorescence signal upon washings (Fig. 7C, dashed green bars), thus assuming the washing cycles did not interfere with the polymer itself. This is crucial to avoid deformation of the recognition cavities regarding size, shape, and chemical functionality towards the target biomolecule.
We studied the effect of ultrasonication duration, power, and pulse mode on de-agglomeration of the QDs nanoparticles dispersed in the cellulose hydrogel, where the maximum input power of the instrument was 700 W with a frequency of 20 kHz. This protocol was performed to de-agglomerate both conjugate structures and to maximize its fluorescence intensity within the cellulose hydrogel. The stability of the imprinted hydrogels was evaluated. When not in use, the sensors were stored in PBS 10 mM pH 7.2, at 4 °C, protected from light. Its stability was evaluated throughout the days. It can be observed that 98.01% of the initial response remained after fifteen days and 95.78% of the initial response remained after thirty days, which indicated that the developed sensor presents good photoluminescence stability (Fig. 7D). Details on the experiment can be found in the ESM file S2 RESULTS AND DISCUSSION.
FT-IR analysis
The samples were previously dried at 60ºC before measurements. In the FT-IR spectra, the band at 3283.64 cm−1 is attributed to the stretching -OH groups from the cellulose structure (Fig. 2, blue). The peaks at 2937.09, 2905.82 and 2875.41 cm−1 are attributed to symmetrical stretching of the -CH2 groups the C-6 of the anhydrous glucose unit (AGU). The bands at 1731.88 and 1655.13 cm−1 correspond to the vibrations of -CO symmetrical stretching and at 1451.62, 1417.12 cm−1 to the -OH bending of absorbed water. The absorption bands at 1351.54 cm−1 and at 1051.34 cm−1 are attributed, respectively, to the -CH2 and to the -OH bending in the cellulose skeleton. The bands around 1306.23 cm−1 and 1274.41 cm−1 are allocated, respectively, to the -CH and -CO in-plane bending of (AGU). In particular, the absorption band at 1274.41 cm−1 is related to -CO anti-symmetric bridge stretching. The bands at 932.13 cm−1 and 848.15 cm−1 derive from the β-glucosidic linkages between the glucose units in the cellulose structure [35, 36]. In the FTIR spectra of the QDs (Fig. 2, orange), the bands at 1564.33 and 1400.22 cm−1 are attributed, respectively, to the symmetric and asymmetric stretching vibrations of the carboxylic groups of the surface of QDs which are in the form of carboxylate anions COO−, attending to the buffer used (PBS 10 mM, pH 6.0) [37, 38]. The presence of OH stretching is also evidenced by the broad peak at 3420.62 cm−1 (Fig. 2, orange). In the spectra of the HEC:MIP@QDs after washings, the band at 2956.25 cm−1 is attributed to the CH2 stretching of the polymer matrix. The band at 1719.22 cm−1 is assigned to the C = O vibrations from the trimethylpropane trimethacrylate crosslinker present in both HEC:MIP@QDs (Fig. 2, red) and HEC:MIPCA19-9@QDs (Fig. 2, pink) and in the non-imprinted HEC:NIP@QDs (Fig. 2, light green). Moreover, both imprinted and non-imprinted samples show peaks around 1636.38 cm−1, attributed to the C–C stretching modes, and peaks at 1319.83 and 1451.84 cm−1, corresponding to the stretching of C = C groups residues of vinylic unsaturation (Fig. 2, light green, red, and pink). The bands at 1151.35 cm−1, 1075.90 cm−1 and 944.09 cm−1 in both conjugates are assigned to the C–O–C vibrations of the polymeric matrix (Fig. 2, light green, red and pink). The weak protuberances at 2954.05 cm−1 (Fig. 2, red), as well as in the protein, (Fig. 2, dark green), may be attributed to the C-H anti-symmetric stretching from CH3 groups the residues of protein probably entrapped inside the polymer, as it also appears in the protein spectrum. Moreover, the band at 3276.66 cm−1 in the protein spectra, is associated with N–H vibrations, which comprise the peptide linkages in proteins. The C = O stretching from the amide groups (CONH) appears at 1648.82 cm−1, the bands associated with C-N stretching appear at 1540.20 cm−1 and at 1520.91 cm−1 while the moderate bands at 1395.66 cm−1 and at 1473.21 cm−1 are associated with the COO− stretching from ester and carboxylate groups (Fig. 2, dark green). The small protein band at 1268.46 cm−1 arises from the N–H bending and the C-N stretching [39]. Some of these bands are also visible to a minor extent in the imprinted HEC:MIP@QDs (Fig. 2, red) without protein and to a major extent in the HEC:MIPCA19-9@QDs (Fig. 2, pink) with the imprinted protein, but not in the non-imprinted hydrogels HEC:NIP@QDs (Fig. 2, light green) at 1557.41 cm−1, 1405.50 cm−1 which may be attributed to the presence of protein residues in the polymer matrix.
FT-IR spectra of the cellulose hydrogels prepared. Cellulose hydrogel (blue); CdTe@MPA QDs (orange); imprinted polymers after polymerization and with the protein, MIPCA19-9@QDs (pink); imprinted cellulose hydrogels after washing and without protein, HEC:MIP@QDs (red); non-imprinted cellulose hydrogels after washing, HEC:NIP@QDs (light green); protein CA19-9 (dark green)
Raman analysis
The bands in the region 2900–3100 cm−1 of the cellulose hydrogel are attributed to the CH stretching of the -CH and the -CH2 groups from the glucose units (Fig. 3, dark green). The C–O–C stretching modes assigned to cellulose ethers appear at 1447.32 cm−1 and 849.52 cm−1, the bands at 1273.44, 1412.39 cm−1 and 1447.65 cm−1 correspond to methylene rock and wag vibrations. The bands at 945.27 cm−1 correspond to the C–C stretching of alkyl side chains of AGU [4]. The C = O stretching modes of AGU units appear at 1628.90 cm−1 and 1732.54 cm−1. The stretching modes of the CH and CH3 groups appear at 3079.43 cm−1, 2958.67 cm−1 and 2920.31 cm−1 [7]. In both imprinted and non-imprinted hydrogels, the bands from (1090.32—1122.43) cm−1 are assigned to the C–C stretching and at 1294.37 cm−1 are assigned to CH2 twisting and wagging from the polymeric matrix (Fig. 3, red, blue, and green). The bands between (3080.23—2923.14) cm−1 are assigned to the combined –CH2 and –CH3 asymmetric stretching bonds from the polymeric matrix and are also present in both imprinted and non-imprinted hydrogels (Fig. 3, red, blue, and green). Regarding the protein spectra (Fig. 3, orange), the NH stretching appears at 3080.83 cm−1, the CH stretching appears at 2956.09 cm−1 and the bands from (2879.95—2845.82) cm−1 are assigned to the CH2 and CH3 stretching from the side chains of the amino acids constituents [40]. The band at 2722.47 cm−1 in the spectra of protein CA19-9 correlates to the stretching vibrations of NH, and OH groups. The bands between (1439.09 – 1461.08) cm−1 correlate to the CN stretching and the bands between (1061.63 – 1131.88) cm−1 correlate to the N–H bending [41]. They are visible in the protein spectra (Fig. 3, orange), and in both imprinted hydrogels HEC:MIPsCA19-9@QDs with protein (Fig. 3, red) and without protein HEC:MIP@QDs (Fig. 3, blue), but not in the spectra of the non-imprinted hydrogels HEC:NIP@QDs (Fig. 3, light green).
Raman spectra of the cellulose hydrogels prepared. Cellulose hydrogel (dark green); imprinted materials after polymerization and with protein, MIPCA19-9@QDs (red); imprinted cellulose hydrogels after washing and without protein, HEC:MIP@QDs (dark blue); non-imprinted cellulose hydrogels after washing, HEC:NIP@QDs (light green); protein CA19-9 (orange)
SEM, EDS, TEM and NTA analysis
SEM images shown in Figure S2_A reveal that the imprinted cellulose hydrogels HEC:MIP@QDs present a polymeric structure with a spherical shape whose diameter could not be precisely determined attending the presence of agglomerates in the polymeric matrix. Nonetheless, both materials appear to have a similar average size diameter, as confirmed by the NTA analysis described below. The correspondent EDS analysis reveals that the element carbon is present in a lower concentration in the imprinted hydrogels, probably due to the more hollowed structure corresponding to the vacant imprinted cavities that remained after the protein removal. On the contrary, the concentration of the element oxygen is slightly higher in the imprinted hydrogels HEC:MIP@QDs relative to the non-imprinted ones HEC:NIP@QDs (Figure S2_B) probably due to the presence of residues of protein that may have not been completely removed from the polymeric matrix during the washings. The bright spots appearing in both images represent the QDs that are spread around the polymer but forming aggregates. This was consistent with the EDS analysis on the bright spots, revealing the presence of the elements cadmium and telluride that are spread around the polymer surface.
The TEM images shown in Figure S3A correspond to the red-emitting QDs nanoparticles presenting a spherical shape but with a tendency to form aggregates. This is also visible in the imprinted hydrogels HEC:MIP@QDs (Figure S3B) and in the non-imprinted hydrogels HEC:NIP@QDs (Figure S3C). The insets in Figure S3A reveal that the size of the nanoparticles could not be precisely determined due to its aggregated state, but estimated to be around 5 nm, attending our previous work [33]. The QDs nanoparticles appear in TEM images as black spots representative of higher electron density materials (Figure S3A). The presence of the polymer matrix is evident around the QDs nanoparticles in both imprinted HEC:MIP@QDs (Figures S3B) and non-imprinted hydrogels HEC:NIP@QDs (Figure S3C). The polymeric material in both hydrogels appears in the TEM images as bulkier and shadowed structures, presenting less contrast relative to the QDs nanoparticles. The bulky structures that appear as zones with lower image contrast are representative of lower electron density materials [42]. As the polymer assembled around the QDs is formed by non-conductive carbon materials, as HEMA and TMPTMA, these carbon materials possess electron densities that are significantly lower than semiconductor materials. For this reason, the polymer layer around the QDs is imaged with less contrast, thus appearing as shadowed zones around the nanoparticles.
NTA is a promising technique because it allows for the visualization of individual nanoparticles, based on the light reflected by the particles in suspension. This analysis was recorded through video capture. The videos and the frames obtained shown in Figure S4 reveal that the shape of both materials MIP- and NIP@QDs was approximately spherical, with an average size diameter of 126.8 nm and 122.9 nm, respectively. The particle concentration, the hydrodynamic diameter, and the size distribution of both MIP- and NIP@QDs determined are shown in Figure S4. The distribution curves of the NTA analysis in ultrapure water, clearly revealed the formation of polydispersed polymeric nanoparticles, being this effect more pronounced in the non-imprinted materials NIP@QDs (Figure S4_c), d) and f)) compared to the imprinted materials MIP@QDs (Figure S4_a), b) and e)). However, no significant differences were observed in the shape and size diameter of both materials, as confirmed by its hydrodynamic diameter of 126.8 ± 1.6 nm for MIP@QDs and 122.9 ± 10.4 nm for NIP@QDs.
AFM analysis
The AFM analysis performed on the cellulose hydrogels with QDs nanoparticles, HEC:QDs (Fig. 4A), revealed a smoother surface (height 2.2 nm) compared with the imprinted hydrogels HEC:MIP@QDs (with height 4.2 nm) (Fig. 4B), and with the non-imprinted hydrogels HEC:NIP@QDs (with height 2.7 nm) (Fig. 4C). This increase in the topography height compared to the HEC:QDs, may be correlated to the presence of the polymer material on the hydrogel. Moreover, the enhanced irregularity observed in the topography of the HEC:MIP@QDs hydrogels compared to the HEC:NIP@QDs is probably attributed to the presence of residues of CA19-9 protein entrapped in the polymer matrix. On the contrary, the empty cavities are responsible for the increase in depth in the HEC:MIP@QDs, within its surface, relative to the HEC:NIP@QDs. Although all cellulose hydrogels present irregular holes, this may be attributed to the water evaporation, leaving behind pores in all cellulose hydrogels [43, 44].
AFM tapping mode images showing 500 nm x 500 nm scan of the cellulose hydrogels prepared. Cellulose hydrogels combined with QDs nanoparticles, HEC:QDs (A); imprinted cellulose hydrogels after washing and without protein, HEC:MIP@QDs (B); non-imprinted cellulose hydrogels after washing, HEC:NIP@QDs (C)
Widefield deconvolution microscopy analysis
Widefield deconvolution microscopy analysis was used to evaluate the HEC:QDs hydrogels (Fig. 5A), the non-imprinted HEC:NIP@QDs (Fig. 5B) and the imprinted hydrogel sensors HEC:MIP@QDs (Fig. 5C). The analysis was performed with the materials in the dried state. All structures show aggregation of the QDs nanoparticles to some extent, which may be caused by the drying of the gels. This fact may contribute to the formation of a more compact polymeric structure within the dried materials. The tightly glomerular structure of the QDs (Fig. 5A) impossibilities the determination of its average size diameter. This measure is compromised in the prepared hydrogels (Fig. 5B and C), where higher aggregation of the nanoparticles is observed. It is possible to clearly identify differences in the conformational microstructure on the non-imprinted (Fig. 5B) and on the imprinted hydrogels (Fig. 5C). The latter ones evidence a bulkier globular structure relative to the former. In addition, the hollowed structure seen in the hydrogels is more pronounced in the imprinted HEC:MIP@QDs (Fig. 5C), relative to the non-imprinted HEC:NIP@QDs (Fig. 5B). This may be attributed to the presence of empty cavities, upon removal of the protein from the polymeric matrix.
Widefield deconvolution microscopy images of the surface of the cellulose hydrogels prepared. The QDs signal is displayed with a “red” (LUT). Cellulose hydrogels combined with QDs nanoparticles, HEC:QDs (A); Non-imprinted cellulose hydrogels after washing, HEC:NIP@QDs (B); Imprinted cellulose hydrogels after washing and without protein, HEC:MIP@QDs (C). The same histogram adjustments were applied for all samples, and images are displayed with a “red” (LUT)
Calibration studies
The imprinted hydrogels prepared HEC:MIP@QDs, as well as the QDs nanoparticles resuspended in the cellulose hydrogel, were calibrated with CA19-9 protein standards in the interval range [9.90 × 10–3 – 1.76 × 103] U/ml. The calibrations were performed in PBS 10 mM pH 6.0 and in a 1000-fold diluted human serum in PBS 10 mM 6.0. Details on the calibration studies of the imprinted hydrogels can be found in the ESM file S2. RESULTS AND DISCUSSION.
Calibrations in PBS
The fluorescence signal of the imprinted hydrogels upon incubation of a standard solution of CA19-9 10.1 U/ml is stabilized after 20 min as seen in Fig. 7A. For this reason, this was the time of incubation selected to perform the calibration studies. Replicates were prepared, and the correspondent calibration curves are shown in Figure S5, upon incubation of each standard solution for 20 min. r.t. The analytical data is represented in Table S1 and analysed according to the Stern–Volmer Eq. (1), where F0 and F are the fluorescence intensities in the absence and in the presence of CA19-9 protein, respectively, kSV is the Stern–Volmer constant, and [Q] is the concentration of CA19-9 protein loaded in the cellulose hydrogel.
A linear correlation in the fluorescence quenching (F/F0) was observed with the QDs nanoparticles and with both imprinted HEC:MIP@QDs (Fig. 6A) and non-imprinted HEC:NIP@QDs hydrogels (Fig. 6B). The imprinting factor (IF) is affected by the site accessibility and mass-transfer resistance of the analyte molecule through the polymer matrix, and is evaluated by the following equation:
Fluorescence emission spectra of the cellulose hydrogels prepared upon calibrations with CA19-9 standards in PBS 10 mM pH 6.0. Imprinted cellulose hydrogels, HEC:MIP@QDs (A); non-imprinted cellulose hydrogels HEC:NIP@QDs (B). MIP@QDs were previously prepared by surface imprinting of 1kU of CA19-9 protein in PBS 10 mM, pH 6.0. Insets are the correspondent Stern–Volmer plots
During calibrations with the QDs nanoparticles, a decrease in the fluorescence signal was observed with increasing concentrations of CA19-9 prepared in PBS 10 mM, pH 6.0 (Figure S5-i) – S5-iii)). This is indicative of the sensing capability of the QDs for CA19-9 protein but evidences a lack of selectivity for the analyte of interest, as the decrease of the signal is continuous during all concentration ranges [9.90 × 10–3 – 1.76 × 103] U/ml (Fig. 6A). The imprinted cellulose hydrogels presented the best analytical response (Figure S5-i) – S5-iii)), relative to the non-imprinted hydrogels (Figure S5-iv) – S5-vi)) and to the QDs nanoparticles (Figure S5-i) – S5-iii)), as shown in Table S1. The best performance of the imprinted hydrogels exhibited a limit of detection (LOD) of 5.83 × 10–4 U/ml (S/N = 3), which is the concentration to quench three times the standard deviation of the blank divided by the slope, an IF of 2.29 and a kSV of -0.1445 (Fig. 6A, Table S1). The interval of linearity is [2.76 × 10–2 – 3.63 × 101] U/ml, which is below the cut-off value for pancreatic cancer diagnosis (CA19-9 > 37 U/ml) [2]. The non-imprinted hydrogels did not present a significant variation in its fluorescence signal upon calibrations with CA19-9 standards in PBS 10 mM pH 6.0 (Fig. 6B, Table S1). This may be attributed to the non-specific adsorption of the template molecules at the polymer surface. The best performance of the non-imprinted hydrogels presents a LOD of 3.93 × 10–2 U/ml (S/N = 3) and a kSV of -0.0630 (Figures S5-iv) – S5-vi)).
Calibrations in a 1000-fold diluted human serum
This study provides concise information of what would happen in a real scenario application, with the existence of other proteins in the human serum. For this purpose, we used Cormay serum HN, which is a lyophilised serum of human origin, designed for routine quality control of inorganic, organic and enzymatic constituents within the normal range. This serum needed to be 1000-fold diluted in PBS 10 mM pH 6.0, to avoid the significant quenching of the fluorescence signal of the QDs nanoparticles. This result was observed when the QDs nanoparticles were resuspended in the human serum in up to 100-fold dilution. The CA19-9 standards were prepared in the diluted human serum, in the interval range of [9.90 × 10–3 – 1.76 × 103] U/ml. During calibrations, each standard was incubated for 20 min. r.t. The calibration curves are shown in Figure S6 and analysed according to Eq. (1). The results are shown in Table S2. A linear correlation in the fluorescence quenching (F/F0) was observed with the QDs nanoparticles (Figure S6-i) – S6-iii)) and with both imprinted HEC:MIP@QDs (Figure S6-i) – S6-iii)) and non-imprinted hydrogels HEC:MIP@QDs (Figure S6-iv) – S6-vi)). The imprinted cellulose hydrogels present the best analytical response compared with the correspondent non-imprinted hydrogels and with QDs nanoparticles (Table S2). This result evidences the superior sensing ability of the imprinted hydrogels towards the target analyte, compared to the QDs nanoparticles. This may be explained by the fact that the imprinted cavities contain functional groups that interact with the complementary groups of the template, through non-covalent bonds. These features, along with the imprinted shape of the template, provide the recognition pattern and the selectivity of the MIP@QDs towards the imprinted molecule. In addition, the narrower linear range of response observed with the imprinted hydrogels is indicative that the recognition process is controlled by binding affinities established through non-covalent bonds between the template molecules and the functional groups inside the imprinted cavities. In opposition, a non-specific response is presented with the QDs nanoparticles and the non-imprinted hydrogels, as shown in Table S2. The best performance of the imprinted hydrogels exhibited an IF of 1.76, a LOD of 1.58 × 10–3 U/ml (S/N = 3), and a kSV of -0.1245 (Figure S7, Table S2). The interval of linearity obtained is [2.76 × 10–2 – 5.23 × 102] U/ml, which is shorter compared to the calibrations performed in PBS 10 mM pH 6.0. In addition, there is a slight decrease in the imprinting factor, which may be influenced by the presence of serum constituents that may adsorb at its surface through non-specific binding. The correspondent non-imprinted hydrogels are subjected to the same effect. Indeed, a slower decrease of its fluorescence signal was observed with increasing concentrations of the target, The best LOD is 1.73 × 10–2 U/ml (S/N = 3), and the correspondent kSV is -0.1144 (Figure S7, Table S2). As for the calibrations with the QDs nanoparticles, a continuous decrease in the fluorescence signal in all concentration ranges was also observed. This result reinforces the absence of selectivity of the QDs towards the target analyte, compared to the selectivity observed when the nanoparticles are conjugated to MIP@QDs (Figure S6).
Selectivity of the imprinted hydrogels
Under the optimized conditions, the analytical performance of imprinted hydrogels was evaluated via response to four different target molecules including creatinine (CREA), C-reactive protein (CRP), carcinoembryonic antigen (CEA) and human serum albumin (HSA).
The results are shown in Fig. 7E, and the corresponding relative errors were calculated (S/N = 3). When CA19-9 was incubated in the imprinted hydrogels HEC:MIP@QDs, a demarked decrease in the fluorescent.
Adsorption kinetics of the imprinted cellulose hydrogels prepared upon different times of incubation with 10.1 U/ml of CA19-9 in PBS 10 mM pH 6.0 (A); Optimizations of the conditions used in the synthesis of the imprinted materials MIP@QDs. Influence of the pH and of the imprinting concentration of CA19-9 (B); Influence of the crosslinker and the time of polymerization (C); Stability of the imprinted cellulose hydrogels HEC:MIP@QDs, upon storage at r.t. (D); Selectivity studies of HEC:MIP@QDs, in the presence of another human serum constituent (E) Error bars: SD n = 3
Upon incubation of the imprinted hydrogels with a solution of CA19-9 10.1 U/ml, the fluorescence intensity decreased to 56% of its initial intensity (Fig. 7E). This result evidence that the imprinted hydrogels possess the highest binding recognition for the target biomarker CA19-9, as the fluorescence quenching was maximum. In the presence of creatinine (CREA), C-reactive protein (CRP) and carcinoembryonic antigen (CEA), there was a slight deviation from the signal with CA19-9 alone whereas, in the presence of human serum albumin (HSA), this deviation is higher, but within the range of the relative errors (Fig. 7E). Except for creatinine, which is a small molecule and so the probability of being captured by the cavities is minimal, the effect of the other proteins assayed was negligible in general. However, in the mixture CA19-9/HSA, the fluorescence intensity was enhanced, with an increase of up to 8%, which was observed compared to the signal of HEC:MIP@QDs in the presence of CA19-9 alone (Fig. 7E). One possible explanation is related to the fact that the protein HSA has a very high solubility in aqueous media, even when the pH of the solution is closer to its isoelectric point, which varies between 5.7 and 5.8, according to theoretical calculations [45]. Another possibility is related to the zeta potential found for HSA suspended in PBS 10 mM pH 6.0, which was -25.76 ± 3.80) mV. The negative charge of the surface of HSA may cause an enhancement of the signal of the imprinted hydrogels HEC:MIP@QDs, as the QDs nanoparticles are negatively charged in PBS 10 mM pH 6.0, in accordance with our work [33].In spite the zeta potential of CEA was not determined experimentally, the isoelectric point is estimated to be around 4.7 [46] thus, it was assumed the surface charge to be negative in the conditions tested. However, a slight decrease of the fluorescence signal was observed instead, which could be explained using the same analogy as for HSA, that the solubility of CEA maybe not in its maximum in PBS 10 mM pH 6.0. Compared to the four interferents, the non-imprinted cellulose hydrogel showed hardly any difference in its fluorescence quenching, showing no selectivity for CA19-9 and no benefit from the recognition process relative to other interfering molecules, as seen in Fig. 7E. Details on the protocol can be found in the ESM file S2. RESULTS AND DISCUSSION.
Finally, the performance of the imprinted hydrogel sensors HEC:MIP@QDs was compared with other sensors reported in the literature for the detection of CA19-9 cancer biomarker (Table 1). The imprinted hydrogels sensor is not as time-consuming as other materials (including incubation of the sample and signal acquisition) but shows remarkable detection efficiency. Though the sensitivity of the hydrogel sensor is not as high as the immune methods, as an antigen-free method, the imprinted hydrogel sensor greatly reduces the cost of analysis and maximizes the efficiency of CA19-9 determinations.
Sample analysis
To further investigate the applications of HEC:MIP@QDs in real sample testing, replicates of the imprinted cellulose hydrogels were suspended in a 1000-fold diluted human serum sample. The proposed hydrogel imprinted sensor was assayed with four concentration levels of CA19-9 that were used to spike a blank sample. Cormay Serum HN 1000-fold diluted in PBS 10 mM pH 6.0 was used as the blank sample. The average recovery and RSD values obtained are listed in Table 2. Details on the experiment can be found in the ESM file S2. RESULTS AND DISCUSSION. The recovery rates ranged from 91.9 to 101.6%, and its RSD was 2.0—5.8%. These results show that the proposed hydrogel sensor was reliable for the analysis of CA19-9 in 1000-fold diluted human serum and could provide optical visualization for early-stage tumour detection. Nonetheless, it would be interesting to compare these results with an established ELISA method for the CA19-9 biomarker or with an HPLC method. Those results may constitute further improvements to the work developed here.
Conclusions
To summarize, a successfully imprinted hydrogel sensor was designed and developed to perform the sensitive and specific monitoring of CA19-9. The work developed demonstrates the ability of the imprinted materials MIP@QDs to be assembled over biocompatible substrates as cellulose. The imprinting concentration, the pH of imprinting and the removal conditions used in the preparation of the imprinted materials MIP@QDs played an important role in the overall performance of the sensor. The validation and acquisition of the imprinted materials is the key to the construction of the imprinted hydrogel sensors. The imprinted hydrogel sensors HEC:MIP@QDs developed showed low LOD, excellent selectivity, good reproducibility, and stability. The results obtained in a 1000-fold diluted human serum evidence the potential of the to be applied as a sensitive and selective molecular recognition sensor for the CA19-9 protein cancer biomarker.
References
Crulhas BP et al (2021) Review—recent advances based on a sensor for cancer biomarker detection. ECS J Solid State Sci Technol 10(4):047004
Azizian A et al (2020) CA19-9 for detecting recurrence of pancreatic cancer. Sci Rep 10(1):1332
Jimenez-Luna C et al (2018) Proteomic biomarkers in body fluids associated with pancreatic cancer. Oncotarget 9(23):16573–16587
Goh SK et al (2017) Serum carbohydrate antigen 19–9 in pancreatic adenocarcinoma: a mini review for surgeons. ANZ J Surg 87(12):987–992
Singhi AD et al (2019) Early detection of pancreatic cancer: opportunities and challenges. Gastroenterology 156(7):2024–2040
Lakemeyer L et al (2021) Diagnostic and prognostic value of CEA and CA19–9 in colorectal cancer. Diseases 9(1)
Serdarevic N (2018) The Comparison between different immunoassays for serum carbohydrate antigen (CA 19–9) concentration measurement. Acta Inform Med 26(4):235–239
Anastasi E et al (2016) Evaluation of an automated CLIA system for the determination of carbohydrate antigen 19–9. Anticancer Res 36(10):5281–5285
Luo Z et al (2018) Near-infrared light-excited core-core-shell UCNP@Au@CdS upconversion nanospheres for ultrasensitive photoelectrochemical enzyme immunoassay. Anal Chem 90(15):9568–9575
Kalyani T et al (2021) Bio-nanocomposite based highly sensitive and label-free electrochemical immunosensor for endometriosis diagnostics application. Bioelectrochemistry 139:107740
Mic M et al (2020) Sensing and interaction of his-tagged CA19-9 antigen with graphene-modified electrodes. Chemosensors 8(4):112
Lv S et al (2018) Wet NH3-triggered NH2-MIL-125(Ti) structural switch for visible fluorescence immunoassay impregnated on paper. Anal Chem 90(24):14121–14125
Bahari D, Babamiri B, Salimi A (2020) An eco-friendly MIP-solid surface fluorescence immunosensor for detection of CA 19–9 tumor marker using Ni nanocluster as an emitter labels. J Iran Chem Soc 17(9):2283–2291
Han X et al (2020) Near-infrared photothermal immunoassay for pancreatic cancer biomarker CA 19–9 on a digital thermometer. Anal Chim Acta 1098:117–124
Jawad ZAR et al (2017) Highly sensitive plasmonic detection of the pancreatic cancer biomarker CA 19–9. Sci Rep 7(1):14309
Chen L, Xu S, Li J (2011) Recent advances in molecular imprinting technology: current status, challenges and highlighted applications. Chem Soc Rev 40(5):2922–2942
Whitcombe MJ, Kirsch N, Nicholls IA (2014) Molecular imprinting science and technology: a survey of the literature for the years 2004–2011. J Mol Recognit 27(6):297–401
Kecili R, Hussain CM (2018) Recent progress of imprinted nanomaterials in analytical chemistry. Int J Anal Chem 2018:8503853
Meng L, Turner APF, Mak WC (2020) Soft and flexible material-based affinity sensors. Biotechnol Adv 39:107398
Xu J, Miao H, Wang J, Pan G (2020) Molecularly imprinted synthetic antibodies: from chemical design to biomedical applications. Small 1906644:1906644–1906665
Alarfaj NA, El-Tohamy MF, Oraby HF (2018) CA 19–9 pancreatic tumor marker fluorescence immunosensing detection via immobilized carbon quantum dots conjugated gold nanocomposite. Int J Mol Sci 19(4)
Lee Y, Kim SS, Lee JH (2019) Chemiluminescent dual-enzyme immunoassays capable of simultaneously quantifying carbohydrate antigen 19–9 and carcinoma embryonic antigen in a sample. Anal Chim Acta 1060:88–96
Wang M et al (2019) Bimetallic cerium and ferric oxides nanoparticles embedded within mesoporous carbon matrix: Electrochemical immunosensor for sensitive detection of carbohydrate antigen 19–9. Biosens Bioelectron 135:22–29
Li J et al (2018) Does polysaccharide is an idea template selection for glycosyl imprinting? Biosens Bioelectron 99:438–442
Piloto AML et al (2020) Label-free quantum dot conjugates for human protein IL-2 based on molecularly imprinted polymers. Sens Actuators, B Chem 304:127343–127354
Carneiro LPT et al (2021) A passive direct methanol fuel cell as transducer of an electrochemical sensor, applied to the detection of carcinoembryonic antigen
Dong C et al (2021) Molecularly imprinted polymers by the surface imprinting technique. Eur Polym J 145:110231
Gomes RS et al (2021) Plastic antibody of polypyrrole_multiwall carbon nanotubes on screen-printed electrodes for cystatin c detection _ enhanced reader
Gonçalves MDL et al (2021) Electrochemical point-of care (PoC) determination of interleukin-6 (IL-6) using a pyrrole (Py) molecularly imprinted polymer (MIP) on a carbonscreen printed electrode (C-SPE)
Tandale P et al (2021) Fluorescent quantum dots: an insight on synthesis and potential biological application as drug carrier in cancer. Biochem Biophys Rep 26:100962
Billah SMR et al (2018) Cellulose-Based Hydrogel for Industrial Applications. In: Mondal M (ed) Cellulose-Based Superabsorbent Hydrogels. A Reference Series, Polymers and Polymeric Composites, pp 1–41
Gui R, Jin H (2019) Recent advances in synthetic methods and applications of photo-luminescent molecularly imprinted polymers. J Photochem Photobiol C Photochem Rev 41:100315
Piloto AM et al (2018) Plastic antibodies tailored on quantum dots for an optical detection of myoglobin down to the femtomolar range. Sci Rep 8(1):4944
Piloto AML et al (2021) Imprinted fluorescent cellulose membranes for the on-site detection of myoglobin in biological media. ACS Appl Bio Mater 4(5):4224–4235
Langkilde FW, Svantesson A (1995) Identification of celluloses with Fourier-transform (FT) mid-infrared, FT-Raman and near-infrared spectrometry. J Pharm Biomed Anal 13(4–5):409–414
Orhan B et al (2018) Synthesis of hydroxyethyl cellulose from industrial waste using microwave irradiation. Sustain Environ Res 28:403–411
Wang Q, Liu P, Zhou X, Zhang X, Fang T, Liu P, Min X, Li X (2012) Thermodynamic and conformational investigation of the influence of CdTe QDs size on the toxic interaction with BSA. J Photochem Photobiol A Chem 230:23–30
Silva FO et al (2012) Effect of surface ligands on the optical properties of aqueous soluble CdTe quantum dots. Nanoscale Res Lett 7(1):536–545
Caine S et al (2012) The application of Fourier transform infrared microspectroscopy for the study of diseased central nervous system tissue. Neuroimage 59(4):3624–3640
Rygula A et al (2013) Raman spectroscopy of proteins: a review. J Raman Spectrosc 44(8):1061–1076
Huang N et al (2011) Full range characterization of the Raman spectra of organs in a murine model. Opt Express 19(23):22892–22909
Utsunomiya S, Ewing RC (2003) Application of high-angle annular dark field scanning transmission electron microscopy, scanning transmission electron microscopy-energy dispersive x-ray spectrometry, and energy-filtered transmission electron microscopy to the characterization of nanoparticles in the environment
Plieva F, Huiting X, Galaev IY, Bergenstahl B, Mattiasson B (2006) Macroporous elastic polyacrylamide gels prepared at subzero temperatures: control of porous structure. J Mater Chem 16:4065–4073
Sobiech M, Maciejewska D, Luliński P (2020) Synthesis and characterization of poly(methacrylic acid-co-trimethylolpropane trimethacrylate) imprinted sorbent for analysis of biogenic amines. Mater Today Commun 22:100739
Olsson MHM et al (2011) PROPKA3: consistent treatment of internal and surface residues in empirical pKa predictions. J Chem Theory Comput 7:525–537
Casey BJ, Kofinas P (2008) Selective binding of carcinoembryonic antigen using imprinted polymeric hydrogels. J Biomed Mater Res A 87(2):359–363
Mo G et al (2021) Spatially-resolved dual-potential sandwich electrochemiluminescence immunosensor for the simultaneous determination of carbohydrate antigen 19–9 and carbohydrate antigen 24–2. Biosens Bioelectron 178:113024
Jiang J et al (2021) Nonmetallic SERS-based immunosensor byintegrating MoS2 nanoflower and nanosheet towards the direct serum detection of carbohydrate antigen 19–9. Biosens Bioelectron 193:113481
Acknowledgements
The authors acknowledge the financial support through project 3C´s (PTDC/AAG TEC/5400/2014). Ana Margarida L. Piloto also acknowledges Fundação para a Ciência e Tecnologia, I.P., for the grant reference SFRH/BPD/116067/2016, co-funded by Programa Operacional Regional Norte 2020, from the European Union. David S. M. Ribeiro thanks FCT (Fundação para a Ciência e Tecnologia) for funding through program DL 57/2016 Norma transitória. The authors acknowledge also the support of i3S Scientific Platforms Advanced Light Microscopy and HEMS, members of the national infrastructure PPBI—Portuguese Platform of Bioimaging (PPBI-POCI-01-0145-FEDER-022122).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Piloto, A.M.L., Ribeiro, D.S.M., Rodrigues, S.S.M. et al. Cellulose-based hydrogel on quantum dots with molecularly imprinted polymers for the detection of CA19-9 protein cancer biomarker. Microchim Acta 189, 134 (2022). https://doi.org/10.1007/s00604-022-05230-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05230-8