Abstract
A fluorometric assay was introduced to determine Acinetobacter baumannii (A. baumannii) in blood samples by utilizing Zr-MOFs both as functional coating for magnetic Fe3O4 nanoparticles to provide modification surface (Zr-mMOF) and as fluorescein carrier to produce fluorescence signals (F@UIO-66-NH2). Through strong Zr-O-P bonding, two distinct terminal phosphate-labeled A. baumannii and lipopolysaccharide (LPS) specific aptamers were attached onto Zr-MOFs to fabricate the magnetic core-shell capture probe (denoted as Zr-mMOF-p-Ab-Apt) and signal probe (denoted as F@UIO-66-NH2-p-LPS-Apt), respectively. After successive incubation with A. baumannii in blood samples and magnetic separation, the sandwich-type composite of capture probe/A. baumannii cells/signal probe was treated with high concentration of anionic phosphate ions to destroy the nano-structure of UIO-66-NH2 in the signal probe and fast release of fluorescein to produce amplified fluorescence signals. Due to the high aptamer modification efficiency of Zr-mMOF-p-Ab-Apt (up to 93%) and its strong affinity to A. baumannii, the enrichment efficiency of this capture probe has reached to 96.7%. Further, due to the high fluorescein loading efficiency of UIO-66-NH2 and our novel amplification strategy to destroy F@UIO-66-NH2-p-LPS-Apt to release and amplify fluorescein signals at 512 nm in the presence of high concentration of anionic phosphate ions, the sensitivity of this method has reached 10 cfu mL−1. This method allows enrichment and determination of A. baumannii within ~2.5 h. The limit of detection of A. baumannii in blood samples is 10 cfu mL−1 with a linear range of 101–105 cfu mL−1. This indicates the potential of this assay for diagnosis of bloodstream infection in early stage.

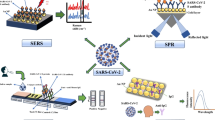
Schematic representation of sandwich–type fluorometric assay for Acinetobacter baumannii in blood samples with the capture probe (Zr-mMOF-p-Ab-Apt) and signal probe (F@UIO-66-NH2-p-LPS-Apt). The limit of detection is down to 10 cfu mL-1 with a linear range of 101-105 cfu mL-1.





Similar content being viewed by others
References
Reinhart K, Daniels R, Kissoon N et al (2017) Recognizing sepsis as a global health priority — a WHO resolution. N Engl J Med 377:414–417. https://doi.org/10.1056/NEJMp1707170
Cleven BEE, Palka-Santini M, Gielen J, Meembor S, Krönke M, Krut O (2006) Identification and characterization of bacterial pathogens causing bloodstream infections by DNA microarray. J Clin Microbiol 44:2389–2397. https://doi.org/10.1128/JCM.02291-05
Ramya M, Khandige D, Ragavendar MS et al (2016) A rapid , highly sensitive and culture-free detection of pathogens from blood by positive enrichment. J Microbiol Methods 131:105–109. https://doi.org/10.1016/j.mimet.2016.10.008
Wellinghausen N, Wirths B, Franz AR et al (2004) Algorithm for the identification of bacterial pathogens in positive blood cultures by real-time LightCycler polymerase chain reaction (PCR) with sequence-specific probes. Diagn Microbiol Infect Dis 48:229–241. https://doi.org/10.1016/j.diagmicrobio.2003.11.005
Lehmann LE, Hunfeld KP, Emrich T, Haberhausen G, Wissing H, Hoeft A, Stüber F (2008) A multiplex real-time PCR assay for rapid detection and differentiation of 25 bacterial and fungal pathogens from whole blood samples. Med Microbiol Immunol 197:313–324. https://doi.org/10.1007/s00430-007-0063-0
Liu TY, Tsai KT, Wang HH et al (2011) Functionalized arrays of Raman-enhancing nanoparticles for capture and culture-free analysis of bacteria in human blood. Nat Commun 2:538. https://doi.org/10.1038/ncomms1546
Lv D, Jiao H, Dong J, Sheng L, Liu J, Dong H, Su A, Zhang M, Xia Z, Oswald JT, Pang D, Liu J, Ouyang H (2019) Biomimetic octopus-like particles for Ultraspecific capture and detection of pathogens. ACS Appl Mater Interfaces 11:22164–22170. https://doi.org/10.1021/acsami.9b05666
Shen H, Wang J, Liu H, Li Z, Jiang F, Wang FB, Yuan Q (2016) Rapid and selective detection of pathogenic Bacteria in bloodstream infections with Aptamer-based recognition. ACS Appl Mater Interfaces 8:19371–19378. https://doi.org/10.1021/acsami.6b06671
Wang J, Wu H, Yang Y et al (2018) Bacterial species-identifiable magnetic nanosystems for early sepsis diagnosis and extracorporeal photodynamic blood disinfection. Nanoscale 10:132–141. https://doi.org/10.1039/c7nr06373c
Zhong Z, Gao X, Gao R, Jia L (2018) Selective capture and sensitive fluorometric determination of Pseudomonas aeruginosa by using aptamer modified magnetic nanoparticles. Microchim Acta 185:1–8. https://doi.org/10.1007/s00604-018-2914-3
Rasoulinejad S, Latif S, Gargari M (2016) Aptamer-nanobody based ELASA for specific detection of Acinetobacter baumannii isolates. J Biotechnol 231:46–54. https://doi.org/10.1016/j.jbiotec.2016.05.024
Osman DI, El-Sheikh SM, Sheta SM et al (2019) Nucleic acids biosensors based on metal-organic framework (MOF): paving the way to clinical laboratory diagnosis. Biosens Bioelectron 141:111451
Mezenov YA, Krasilin AA, Dzyuba VP et al (2019) Metal–organic frameworks in modern physics: highlights and perspectives. Adv Sci:1900506. https://doi.org/10.1002/advs.201900506
Wang S, McGuirk CM, Ross MB et al (2017) General and direct method for preparing oligonucleotide-functionalized metal-organic framework nanoparticles. J Am Chem Soc 139:9827–9830. https://doi.org/10.1021/jacs.7b05633
Meng HM, Hu XX, Kong GZ, Yang C, Fu T, Li ZH, Zhang XB (2018) Aptamer-functionalized nanoscale metal-organic frameworks for targeted photodynamic therapy. Theranostics 8:4332–4344. https://doi.org/10.7150/thno.26768
Yang J, Dai Y, Zhu X et al (2015) Metal-organic frameworks with inherent recognition sites for selective phosphate sensing through their coordination-induced fluorescence enhancement effect. J Mater Chem A 3:7445–7452. https://doi.org/10.1039/c5ta00077g
Liu D, Lu K, Poon C, Lin W (2014) Metal-organic frameworks as sensory materials and imaging agents. Inorg Chem 53:1916–1924. https://doi.org/10.1021/ic402194c
Ling P, Qian C, Yu J, Gao F (2020) Artificial nanozyme based on platinum nanoparticles anchored metal-organic frameworks with enhanced electrocatalytic activity for detection of telomeres activity. Biosens Bioelectron 149:111838. https://doi.org/10.1016/j.bios.2019.111838
Feng J, Wang H, Ma Z (2020) Ultrasensitive amperometric immunosensor for the prostate specific antigen by exploiting a Fenton reaction induced by a metal-organic framework nanocomposite of type Au/Fe-MOF with peroxidase mimicking activity. Microchim Acta 187. https://doi.org/10.1007/s00604-019-4075-4
Huang Y, Liang R, Yu H et al (2020) Thi-au-Fe3O4 confined in ZIF-8 nanoreactor as signal-amplifying tag for constructing high-efficiency electrochemical platform. Sensors Actuators B Chem 305:127496. https://doi.org/10.1016/j.snb.2019.127496
Wang H, Ma Z (2019) “Off-on” signal amplification strategy amperometric immunosensor for ultrasensitive detection of tumour marker. Biosens Bioelectron 132:265–270. https://doi.org/10.1016/j.bios.2019.03.013
Wu H, Chua YS, Krungleviciute V, Tyagi M, Chen P, Yildirim T, Zhou W (2013) Unusual and highly tunable missing-linker defects in zirconium metal-organic framework UiO-66 and their important effects on gas adsorption. J Am Chem Soc 135:10525–10532. https://doi.org/10.1021/ja404514r
Yang S, Sun J, Wu X, Zhang L (2018) Determinants of Mortality in Patients with Nosocomial Acinetobacter baumannii Bacteremia in Southwest China: A Five-Year Case-Control Study. Can J Infect Dis Med Microbiol:3150965. https://doi.org/10.1155/2018/3150965
Wang N, Dai H, Sai L et al (2019) Copper ion-assisted gold nanoparticle aggregates for electrochemical signal amplification of lipopolysaccharide sensing. Biosens Bioelectron 126:529–534. https://doi.org/10.1016/j.bios.2018.11.021
Qian J, Ren C, Wang C, Chen W, Lu X, Li H, Liu Q, Hao N, Li H, Wang K (2018) Magnetically controlled fluorescence aptasensor for simultaneous determination of ochratoxin A and aflatoxin B1. Anal Chim Acta 1019:119–127. https://doi.org/10.1016/j.aca.2018.02.063
Gao X, Cui R, Ji G, Liu Z (2018) Size and surface controllable metal-organic frameworks (MOFs) for fluorescence imaging and cancer therapy. Nanoscale 10:6205–6211. https://doi.org/10.1039/c7nr08892b
Evans BC, Nelson CE, Yu SS et al (2013) Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive Endosomolytic agents for cytosolic delivery of biomacromolecular drugs. J Vis Exp 73:e50166. https://doi.org/10.3791/50166
Liu Y, Hou W, Xia L, Cui C, Wan S, Jiang Y, Yang Y, Wu Q, Qiu L, Tan W (2018) ZrMOF nanoparticles as quenchers to conjugate DNA aptamers for target-induced bioimaging and photodynamic therapy. Chem Sci 9:7505–7509. https://doi.org/10.1039/C8SC02210K
Bai YL, Shahed-Al-Mahmud M, Selvaprakash K et al (2019) Tail Fiber protein-immobilized magnetic nanoparticle-based affinity approaches for detection of Acinetobacter baumannii. Anal Chem 91:10335–10342. https://doi.org/10.1021/acs.analchem.9b02964
El Ichi S, Leon F, Vossier L et al (2014) Microconductometric immunosensor for label-free and sensitive detection of gram-negative bacteria. Biosens Bioelectron 54:378–384. https://doi.org/10.1016/j.bios.2013.11.016
Bell CS, Ph D, Mejías R et al (2017) Magnetic extraction of Acinetobacter baumannii using Colistin functionalized γ-Fe 2 O 3 / Au Core / Shell composite Nanoclusters. ACS Appl Mater Interfaces 9:26719–26730
Acknowledgments
We thank Dr. Shuguang Lu (PLA, Third Military Medical University, Chongqing, China) for kindly providing the ATCC19606 strains in this study. This research is financially supported by grants from the National Natural Science Foundation of China (N.O. 81802115, 81702101, and 81672112), Chongqing Technology Innovation and Application Demonstration Project (cstc2018jscx-msybX0010), Luzhou Municipal People’s Government & Southwest Medical University Science and Technology Strategic Cooperation Project (N.O. 2018LZXNYD-ZK08), Chongqing Special Postdoctoral Science Foundation (N.O. Xm2017090) and China Postdoctoral Science Foundation (N.O. 2018 M643425).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 4752 kb)
Rights and permissions
About this article
Cite this article
Yang, S., Guo, Y., Fan, J. et al. A fluorometric assay for rapid enrichment and determination of bacteria by using zirconium-metal organic frameworks as both capture surface and signal amplification tag. Microchim Acta 187, 188 (2020). https://doi.org/10.1007/s00604-020-4136-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-4136-8