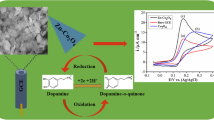
Abstract
A simple binary copper selenide, CuSe nanostructure, has been investigated as electrochemical sensor for dopamine detection. The hydrothermally synthesized and electrodeposited CuSe nanostructures showed high sensitivity for dopamine detection with low limit of detection (LOD). A sensitivity of 26 μA/μM.cm2 was obtained with this electrochemical sensor which is ideal to detect even small fluctuations in the transient dopamine concentration. Apart from high sensitivity and low LOD, the dopamine oxidation on the catalyst surface also occurred at a low applied potential (< 0.18 V vs Ag|AgCl), thereby significantly increasing selectivity of the process specifically with respect to ascorbic and uric acids, which are considered to be the most prominent interferents for dopamine detection. Electrochemical redox tunability of the catalytic Cu center along with low coordination geometry is believed to enhance the rate of dopamine attachment and oxidation on the catalyst surface thereby reducing the applied potential. The presence of Cu also increases conductivity of the catalyst composite which further improves the charge transfer thus increasing the sensitivity of the device. This is the first report of electrochemical dopamine sensing with a simple binary selenide comprising earth-abundant elements and can have large significance in designing efficient sensors that can be transformative for understanding neurodegenerative diseases further.

Graphical abstract






Similar content being viewed by others
References
Aziz A, Asif M, Ashraf G, Azeem M, Majeed I, Ajmal M, Wang J, Liu H (2019) Advancements in electrochemical sensing of hydrogen peroxide, glucose and dopamine by using 2D nanoarchitectures of layered double hydroxides or metal dichalcogenides. A review. Microchim Acta 186, Article no. 671
Salamon J, Sathishkumar Y, Ramachandran K, Lee YS, Yoo DJ, Kim AR, Gnana kumar G (2015) One-pot synthesis of magnetite nanorods/graphene composites and its catalytic activity toward electrochemical detection of dopamine. Biosens Bioelectron 64:269–276. doi:https://doi.org/10.1016/j.bios.2014.08.085
Kaya M, Volkan M (2012) New approach for the surface enhanced resonance Raman scattering (SERRS) detection of dopamine at picomolar (pM) levels in the presence of ascorbic acid. Anal Chem 84 (18):7729–7735. doi:https://doi.org/10.1021/ac3010428
Jie Y, Wang N, Cao X, Xu Y, Li T, Zhang X, Wang ZL (2015) Self-powered triboelectric nanosensor with poly(tetrafluoroethylene) nanoparticle arrays for dopamine detection. ACS Nano 9 (8):8376–8383. doi:https://doi.org/10.1021/acsnano.5b03052
Kim D-S, Kang E-S, Baek S, Choo S-S, Chung Y-H, Lee D, Min J, Kim T-H (2018) Electrochemical detection of dopamine using periodic cylindrical gold nanoelectrode arrays. Sci Rep 8(1):14049. https://doi.org/10.1038/s41598-018-32477-0
Schindler S, Bechtold T (2019) Mechanistic insights into the electrochemical oxidation of dopamine by cyclic voltammetry. J Electroanal Chem 836:94–101. https://doi.org/10.1016/j.jelechem.2019.01.069
Labib M, Sargent EH, Kelley SO (2016) Electrochemical methods for the analysis of clinically relevant biomolecules. Chem Rev 116(16):9001–9090. https://doi.org/10.1021/acs.chemrev.6b00220
Jackowska K, Krysinski P (2013) New trends in the electrochemical sensing of dopamine. Anal Bioanal Chem 405(11):3753–3771. https://doi.org/10.1007/s00216-012-6578-2
Li Y, Lin X (2006) Simultaneous electroanalysis of dopamine, ascorbic acid and uric acid by poly (vinyl alcohol) covalently modified glassy carbon electrode. Sensors Actuators B Chem 115(1):134–139. https://doi.org/10.1016/j.snb.2005.08.022
Liu A, Honma I, Zhou H (2007) Simultaneous voltammetric detection of dopamine and uric acid at their physiological level in the presence of ascorbic acid using poly(acrylic acid)-multiwalled carbon-nanotube composite-covered glassy-carbon electrode. Biosens Bioelectron 23(1):74–80. https://doi.org/10.1016/j.bios.2007.03.019
Muguruma H, Inoue Y, Inoue H, Ohsawa T (2016) Electrochemical study of dopamine at electrode fabricated by cellulose-assisted aqueous dispersion of long-length carbon nanotube. J Phys Chem 120(22):12284–12292. https://doi.org/10.1021/acs.jpcc.6b03715
Jiang L, Nelson GW, Abda J, Foord JS (2016) Novel modifications to carbon-based electrodes to improve the electrochemical detection of dopamine. ACS Appl Mater Interfaces 8(42):28338–28348. https://doi.org/10.1021/acsami.6b03879
Lin C, Chen L, Tanner EEL, Compton RG (2018) Electroanalytical study of dopamine oxidation on carbon electrodes: from the macro- to the micro-scale. Phys Chem Chem Phys 20(1):148–157. https://doi.org/10.1039/C7CP07450F
Chen D, Tian C, Li X, Li Z, Han Z, Zhai C, Quan Y, Cui R, Zhang G (2018) Electrochemical determination of dopamine using a glassy carbon electrode modified with a nanocomposite consisting of nanoporous platinum-yttrium and graphene. Microchim Acta 185:98. https://doi.org/10.1007/s00604-017-2624-2
Savk A, Özdil B, Demirkan B, Nas MS, Calimli MH, Alma MH, Inamuddin AAM, Şen F (2019) Multiwalled carbon nanotube-based nanosensor for ultrasensitive detection of uric acid, dopamine, and ascorbic acid. Mater Sci Eng C 99:248–254. https://doi.org/10.1016/j.msec.2019.01.113
Lakshmi D, Bossi A, Whitcombe MJ, Chianella I, Fowler SA, Subrahmanyam S, Piletska EV, Piletsky SA (2009) Electrochemical sensor for catechol and dopamine based on a catalytic molecularly imprinted polymer-conducting polymer hybrid recognition element. Anal Chem 81(9):3576–3584. https://doi.org/10.1021/ac802536p
Oztekin Y, Tok M, Bilici E, Mikoliunaite L, Yazicigil Z, Ramanaviciene A, Ramanavicius A (2012) Copper nanoparticle modified carbon electrode for determination of dopamine. Electrochim Acta 76:201–207. https://doi.org/10.1016/j.electacta.2012.04.105
Jalali, M., Filine, E., Dalfen, S., Mahshid S. Microscale reactor embedded with graphene/hierarchical gold nanostructures for electrochemical sensing: application to the determination of dopamine. Microchim Acta 187, 90 (2020)
Fang B, Wang G, Zhang W, Li M, Kan X (2005) Fabrication of Fe3O4 nanoparticles modified electrode and its application for voltammetric sensing of dopamine. Electroanalysis 17(9):744–748. https://doi.org/10.1002/elan.200403136
Zhang F, Li Y, Gu YE et al (2011) One-pot solvothermal synthesis of a Cu2O/graphene nanocomposite and its application in an electrochemical sensor for dopamine. Microchim Acta 173:103–109. https://doi.org/10.1007/s00604-010-0535-6
Roy PR, Okajima T, Ohsaka T (2003) Simultaneous electroanalysis of dopamine and ascorbic acid using poly (N,N-dimethylaniline)-modified electrodes. Bioelectrochemistry 59(1):11–19. https://doi.org/10.1016/S1567-5394(02)00156-1
Li Q, Wang Y, Luo G (2000) Voltammetric separation of dopamine and ascorbic acid with graphite electrodes modified with ultrafine TiO2. Mater Sci Eng C 11(1):71–74. https://doi.org/10.1016/S0928-4931(00)00132-6
Masud J, Liyanage WPR, Cao X, Saxena A, Nath M (2018) Copper selenides as high-efficiency electrocatalysts for oxygen evolution reaction. ACS Appl Mater Interfaces 1(8):4075–4083. https://doi.org/10.1021/acsaem.8b00746
Umapathi S, Masud J, Swesi AT, Nath M (2017) FeNi2Se4–reduced graphene oxide nanocomposite: enhancing bifunctional electrocatalytic activity for oxygen evolution and reduction through synergistic effects. Adv Sustain Syst 1(10):1700086. https://doi.org/10.1002/adsu.201700086
Swesi AT, Masud J, Liyanage WPR, Umapathi S, Bohannan E, Medvedeva J, Nath M (2017) Textured NiSe2 film: bifunctional electrocatalyst for full water splitting at remarkably low overpotential with high energy efficiency. Sci Rep 7(1):2401. https://doi.org/10.1038/s41598-017-02285-z
Ma M, Zhu W, Zhao D, Ma Y, Hu N, Suo Y, Wang J (2019) Surface engineering of nickel selenide nanosheets array on nickel foam: an integrated anode for glucose sensing. Sensors Actuators B Chem 278:110–116. doi:https://doi.org/10.1016/j.snb.2018.09.075
Wang Y-H, Huang K-J, Wu X (2017) Recent advances in transition-metal dichalcogenides based electrochemical biosensors: a review. Biosens Bioelectron 97:305–316. doi:https://doi.org/10.1016/j.bios.2017.06.011
Li X (2019) A novel electrochemical sensor based on the synergistic effect of trace platinum and foliate Co0.85Se for the determination of dopamine. Int J Electrochem Sci:4327-4337. doi:https://doi.org/10.20964/2019.05.68
Chia X, Eng AYS, Ambrosi A, Tan SM, Pumera M (2015) Electrochemistry of nanostructured layered transition-metal dichalcogenides. Chem Rev 115(21):11941–11966. https://doi.org/10.1021/acs.chemrev.5b00287
Hammami A, Sahli R, Raouafi N (2016) Indirect amperometric sensing of dopamine using a redox-switchable naphthoquinone-terminated self-assembled monolayer on gold electrode. Microchim Acta 183(3):1137–1144. https://doi.org/10.1007/s00604-015-1739-6
Yadav P, Manivannan S, Kim H-S, Pandey K, Kim K, Kim J (2017) Electrochemical properties of highly sensitive and selective CuO nanostructures based neurotransmitter dopamine sensor. Electroanalysis 29(9):2106–2113. https://doi.org/10.1002/elan.201700195
Sheng Z-H, Zheng X-Q, Xu J-Y, Bao W-J, Wang F-B, Xia X-H (2012) Electrochemical sensor based on nitrogen doped graphene: simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens Bioelectron 34(1):125–131. https://doi.org/10.1016/j.bios.2012.01.030
Masud J, Ioannou P-C, Levesanos N, Kyritsis P, Nath M (2016) A molecular Ni-complex containing tetrahedral nickel selenide core as highly efficient electrocatalyst for water oxidation. ChemSusChem 9(22):3128–3132. https://doi.org/10.1002/cssc.201601054
Chen Y, Tan TC (1994) Selectivity enhancement of an immobilized apple powder enzymatic sensor for dopamine. Biosens Bioelectron 9(6):401–410. https://doi.org/10.1016/0956-5663(94)90027-2
Hou S, Kasner ML, Su S, Patel K, Cuellari R (2010) Highly sensitive and selective dopamine biosensor fabricated with silanized graphene. J Phys Chem C114 (35):14915–14921. doi:https://doi.org/10.1021/jp1020593
De Silva U, Masud J, Zhang N, Hong Y, Liyanage WPR, Asle Zaeem M, Nath M (2018) Nickel telluride as a bifunctional electrocatalyst for efficient water splitting in alkaline medium. J Mater Chem A 6(17):7608–7622. https://doi.org/10.1039/C8TA01760C
Zhang Y, Ji Y, Wang Z, Liu S, Zhang T (2015) Electrodeposition synthesis of reduced graphene oxide–carbon nanotube hybrids on indium tin oxide electrode for simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. RSC Adv 5(129):106307–106314. https://doi.org/10.1039/C5RA24727F
Wang H, Ren F, Wang C, Yang B, Bin D, Zhang K, Du Y (2014) Simultaneous determination of dopamine, uric acid and ascorbic acid using a glassy carbon electrode modified with reduced graphene oxide. RSC Adv 4(51):26895–26901. https://doi.org/10.1039/C4RA03148B
Lian Q, He Z, He Q, Luo A, Yan K, Zhang D, Lu X, Zhou X (2014) Simultaneous determination of ascorbic acid, dopamine and uric acid based on tryptophan functionalized graphene. Anal Chim Acta 823:32–39. https://doi.org/10.1016/j.aca.2014.03.032
Manbohi A, Ahmadi SH (2019) Sensitive and selective detection of dopamine using electrochemical microfluidic paper-based analytical nanosensor. Sens Biosensing Res 23:100270. https://doi.org/10.1016/j.sbsr.2019.100270
Funding
This work was partially supported by the NSF DMR-1710313.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work; there is no professional or other personal interest of any nature or kind in any product, service, and/or company that could be constructed as influencing the position presented in or the review of the manuscript entitled.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 9.60 MB)
Rights and permissions
About this article
Cite this article
Umapathi, S., Masud, J., Coleman, H. et al. Electrochemical sensor based on CuSe for determination of dopamine. Microchim Acta 187, 440 (2020). https://doi.org/10.1007/s00604-020-04405-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04405-5