Abstract
β-Cyclodextrin-functionalized carbon nitride nanosheets were modified with a molecularly imprinted polymer to obtain a fluorescent probe of type MIP@β-CD/CNNS which is shown to enable fluorometric determination of sterigmatocystin (STG). The material was characterized by transmission electron microscopy, infrared spectra, powder X-ray diffraction, X-ray photoelectron spectroscopy, and by absorption and emission spectra. The modified CNNSs have a good fluorescence quantum yield (13%), high sorption capacity for STG (86 mg·g−1), fast adsorption rate (25 min), and superior adsorption selectivity (with an imprint factor 2.56). When used as an optical probe for STG, the CNNSs act as the chromophore, while β-CD and MIP act as the recognition groups. The blue fluorescence of MIP@β-CD/CNNS (with excitation/emission maxima at 368/432 nm) is quenched by STG. Fluorescence drops linearly in the 0.15 to 3.1 μM STG concentration range. The lower detection limit is 74 nM. The method was successfully applied to the determination of STG in spiked wheat extract. Conceivably, this detection scheme based on a combination of β-CD inclusion and molecular imprinting may be extended to the detection of various other organic compounds.

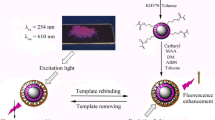
Schematic representation of the preparation of surface-imprinted β-cyclodextrin-functionalized carbon nitride nanosheets. These are used, along with a molecularly imprinted polymer, for fluorometric determination of sterigmatocystin.






Similar content being viewed by others
Change history
08 September 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00604-021-04961-4
References
Zhou X, Yang L, Tan XP, Zhao GF, Xie XG, Du GB (2018) A robust electrochemical immunosensor based on hydroxyl pillar 5 arene@AuNPs@g-C3N4 hybrid nanomaterial for ultrasensitive detection of prostate specific antigen. Biosens Bioelectron 112:31–39. https://doi.org/10.1016/j.bios.2018.04.036
Jing Han HYZ, Gao MX, Huang CZ (2016) A graphitic carbon nitride based fluorescence resonance energy transfer detection of riboflavin. Talanta 148:279–284. https://doi.org/10.1016/j.talanta.2015.10.038
Rahbar N, Salehnezhad Z, Hatamie A, Babapour A (2018) Graphitic carbon nitride nanosheets as a fluorescent probe for chromium speciation. Mikrochim Acta 185:101. https://doi.org/10.1007/s00604-017-2615-3
Wang Q, Wang W, Lei J, Xu N, Gao F, Ju H (2013) Fluorescence quenching of carbon nitride nanosheet through its interaction with DNA for versatile fluorescence sensing. Anal Chem 85:12182–12188. https://doi.org/10.1021/ac403646n
Xiang M-H, Liu J-W, Li N, Tang H, Yu R-Q, Jiang J-H (2016) A fluorescent graphitic carbon nitride nanosheet biosensor for highly sensitive, label-free detection of alkaline phosphatase. Nanoscale 8:4727–4732. https://doi.org/10.1039/c5nr08278a
Del Valle EMM (2004) Cyclodextrins and their uses: a review. Process Biochem 39:1033–1046. https://doi.org/10.1016/s0032-9592(03)00258-9
Lai WF, Rogach AL, Wong WT (2017) Chemistry and engineering of cyclodextrins for molecular imaging. Chem Soc Rev 46:6379–6419. https://doi.org/10.1039/c7cs00040e
Cheng Y, Jiang P, Lin S, Li Y, Dong X (2014) An imprinted fluorescent chemosensor prepared using dansyl-modified β-cyclodextrin as the functional monomer for sensing of cholesterol with tailor-made selectivity. Sensors Actuators B Chem 193:838–843. https://doi.org/10.1016/j.snb.2013.12.039
Lenik J (2017) Cyclodextrins based electrochemical sensors for biomedical and pharmaceutical analysis. Curr Med Chem 24:2359–2391. https://doi.org/10.2174/0929867323666161213101407
Gao JW, Xiong HW, Zhang W, Wang Y, Wang HX, Wen W, Zhang XH, Wang SF (2018) Electrochemiluminescent aptasensor based on beta-cyclodextrin/graphitic carbon nitride composite for highly selective and ultrasensitive assay of platelet derived growth factor BB. Carbon 130:416–423. https://doi.org/10.1016/j.carbon.2018.01.026
Zou YD, Wang XX, Ai YJ, Liu YH, Ji YF, Wang HQ, Hayat T, Alsaedi A, Hu WP, Wang XK (2016) Beta-Cyclodextrin modified graphitic carbon nitride for the removal of pollutants from aqueous solution: experimental and theoretical calculation study. J Mater Chem A 4:14170–14179. https://doi.org/10.1039/c6ta05958a
Li R, Feng Y, Pan G, Liu L (2019) Advances in molecularly imprinting Technology for Bioanalytical Applications. Sensors (Basel) 19:177–210. https://doi.org/10.3390/s19010177
Figueiredo L, Erny GL, Santos L, Alves A (2016) Applications of molecularly imprinted polymers to the analysis and removal of personal care products: a review. Talanta 146:754–765. https://doi.org/10.1016/j.talanta.2015.06.027
Yang Q, Li J, Wang X, Peng H, Xiong H, Chen L (2018) Strategies of molecular imprinting-based fluorescence sensors for chemical and biological analysis. Biosens Bioelectron 112:54–71. https://doi.org/10.1016/j.bios.2018.04.028
Phan NVH, Sussitz HF, Ladenhauf E, Pum D, Lieberzeit PA (2018) Combined layer/particle approaches in surface molecular imprinting of proteins: signal enhancement and competition. Sensors (Basel) 18:180–190. https://doi.org/10.3390/s18010180
Asman S, Mohamad S, Sarih N (2015) Exploiting β-Cyclodextrin in molecular imprinting for achieving recognition of Benzylparaben in aqueous media. Int J Mol Sci 16:3656–3676. https://doi.org/10.3390/ijms16023656
Cheng Y, Jiang P, Dong X (2015) Molecularly imprinted fluorescent chemosensor synthesized using quinoline-modified-β-cyclodextrin as monomer for spermidine recognition. RSC Adv 5:55066–55074. https://doi.org/10.1039/c5ra07761c
Cheng Y, Nie J, Li Z, Yan Z, Xu G, Li H, Guan D (2017) A molecularly imprinted polymer synthesized using beta-cyclodextrin as the monomer for the efficient recognition of forchlorfenuron in fruits. Anal Bioanal Chem 409:5065–5072. https://doi.org/10.1007/s00216-017-0452-1
Xu S, Chen L, Ma L (2018) Fluorometric determination of quercetin by using graphitic carbon nitride nanoparticles modified with a molecularly imprinted polymer. Mikrochim Acta 185:492. https://doi.org/10.1007/s00604-018-3016-y
Lee HJ, Ryu D (2017) Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: public health perspectives of their co-occurrence. J Agric Food Chem 65:7034–7051. https://doi.org/10.1021/acs.jafc.6b04847
Takagi M, Uno S, Kokushi E, Sato F, Wijayagunawardane M, Fink-Gremmels J (2018) Measurement of urinary concentrations of the mycotoxins zearalenone and sterigmatocystin as biomarkers of exposure in mares. Reprod Domest Anim 53:68–73. https://doi.org/10.1111/rda.13054
Zhao Y, Wang Q, Huang J, Ma L, Chen Z, Wang F (2018) Aflatoxin B1 and sterigmatocystin in wheat and wheat products from supermarkets in China. Food Addit Contam Part B Surveill 11:9–14. https://doi.org/10.1080/19393210.2017.1388295
Diaz Nieto CH, Granero AM, Zon MA, Fernandez H (2018) Sterigmatocystin: a mycotoxin to be seriously considered. Food Chem Toxicol 118:460–470. https://doi.org/10.1016/j.fct.2018.05.057
Amadasi A, Dall'asta C, Ingletto G, Pela R, Marchelli R, Cozzini P (2007) Explaining cyclodextrin-mycotoxin interactions using a 'natural' force field. Bioorg Med Chem 15:4585–4594. https://doi.org/10.1016/j.bmc.2007.04.006
Zhang X, Xie X, Wang H, Zhang J, Pan B, Xie Y (2013) Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging. J Am Chem Soc 135:18–21. https://doi.org/10.1021/ja308249k
Wang Y, Wei Z, Luo X, Wan Q, Qiu R, Wang S (2019) An ultrasensitive homogeneous aptasensor for carcinoembryonic antigen based on upconversion fluorescence resonance energy transfer. Talanta 195:33–39. https://doi.org/10.1016/j.talanta.2018.11.011
Zu F, Yan F, Bai Z, Xu J, Wang Y, Huang Y, Zhou X (2017) The quenching of the fluorescence of carbon dots: a review on mechanisms and applications. Microchim Acta 184:1899–1914. https://doi.org/10.1007/s00604-017-2318-9
Liang Z, Kang M, Payne GF, Wang X, Sun R (2016) Probing energy and Electron transfer mechanisms in fluorescence quenching of biomass carbon quantum dots. ACS Appl Mater Interfaces 8:17478–17488. https://doi.org/10.1021/acsami.6b04826
Oplatowska-Stachowiak M, Reiring C, Sajic N, Haasnoot W, Brabet C, Campbell K, Elliott CT, Salden M (2018) Development and in-house validation of a rapid and simple to use ELISA for the detection and measurement of the mycotoxin sterigmatocystin. Anal Bioanal Chem 410:3017–3023. https://doi.org/10.1007/s00216-018-0988-8
Biancardi A, Dall'Asta C (2015) Determination of sterigmatocystin in feed by LC-MS/MS. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 32:2093–2100. https://doi.org/10.1080/19440049.2015.1094709
Liu J-M, Wei S-Y, Liu H-L, Fang G-Z, Wang S (2017) Preparation and evaluation of Core–Shell magnetic molecularly imprinted polymers for solid-phase extraction and determination of Sterigmatocystin in food. Polymers 9:546. https://doi.org/10.3390/polym9100546
Hossain MZ, Goto T (2015) Determination of sterigmatocystin in grain using gas chromatography-mass spectrometry with an on-column injector. Mycotoxin Res 31:17–22. https://doi.org/10.1007/s12550-014-0214-2
Xu L, Fang G, Pan M, Wang X, Wang S (2016) One-pot synthesis of carbon dots-embedded molecularly imprinted polymer for specific recognition of sterigmatocystin in grains. Biosens Bioelectron 77:950–956. https://doi.org/10.1016/j.bios.2015.10.072
Liu J-M, Cao F-Z, Fang G-Z, Wang S (2017) Upconversion nanophosphor-involved molecularly imprinted fluorescent polymers for sensitive and specific recognition of Sterigmatocystin. Polymers 9:299. https://doi.org/10.3390/polym9070299
Acknowledgements
This work was financially supported by Independent innovation fund project of agricultural science and technology of Jiangsu Province in 2017 [No CX (17) 1003].
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1219 kb)
Rights and permissions
About this article
Cite this article
Shi, J., Li, G., Cui, Y. et al. Surface-imprinted β-cyclodextrin-functionalized carbon nitride nanosheets for fluorometric determination of sterigmatocystin. Microchim Acta 186, 808 (2019). https://doi.org/10.1007/s00604-019-3867-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3867-x