Abstract
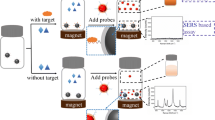
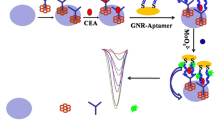
A fluorometric method is described for the determination of the tumor biomarker mucin 1 (MUC1). It is based on signal amplification of the hybridization chain reaction (HCR), and the interaction between a luminescent ruthenium(II) complex and CdZnTeS quantum dots (QDs). If MUC1 bind to the biotin-labeled aptamer, it will initiate HCR with hairpins H1 and H2 to form a long-range dsDNA. The long nucleic acid chains are then linked on the surface of streptavidin-modified magnetic microparticles (MMPs) through streptavidin-biotin interaction. The luminescent ruthenium(II) complex is then embedded in the long dsDNA linked to the MMPs. Hence, there is little Ru complex in the supernatant after magnetic separation, and the fluorescence of the CdZnTeS QDs (best measured at excitation/emission wavelengths of 350/530 nm) is only slightly quenched. In the absence of target, the fluorescence of the CdZnTeS QDs is strongly quenched. Fluorescence increases linearly in the 0.2–100 ng·mL−1 MUC1 concentration range, and the LOD is 0.13 ng·mL−1 (at S/N = 3). The method was applied to the determination of MUC1 in spiked human serum samples.

A fluorometric turn-on aptasensor for mucin 1 is described that is based on the interaction between a Ru(II) complex and quantum dots (QDs). The detection system includes biotin-labeled aptamer-H0, hairpins H1 and H2, streptavidin-modified magnetic microparticles (MMPs), Ru(bpy)2(dppx)2+ and CdZnTeS QDs.






Similar content being viewed by others
References
Donald WK (2009) Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer 9:874–885
Singh R, Bandyopadhyay D (2007) MUC1: a target molecule for cancer therapy. Cancer Biol Ther 6:481–486
Chinen AB, Guan CM, Ferrer JR, Barnaby SN, Merkel TJ, Mirkin CA (2015) Nanoparticle probes for the detection of cancer biomarkers, cells, and tissues by fluorescence. Chem Rev 115:10530–10574
Chikkaveeraiah BV, Bhirde A, Morgan NY, Eden HS, Chen XY (2012) Electrochemical immunosensors for detection of cancer protein biomarkers. ACS Nano 6:6546–6561
Falahat R, Wiranowska M, Gallant ND, Toomey R, Hill R, Alcantar N (2015) A cell ELISA for the quantification of MUC1 mucin (CD227) expressed by cancer cells of epithelial and neuroectodermal origin. Cell Immunol 298:96–103
Feng JJ, Wu XL, Ma W, Kuang H, Xu LG, Xu CL (2015) A SERS active bimetallic core–satellite nanostructure for the ultrasensitive detection of Mucin-1. Chem Commun 51:14761–14763
Wang N, Zhang MM, Chen XJ, Ma XX, Li C, Zhang Z, Tang JL (2017) Mapping the interaction sites of mucin 1 and DNA aptamer by atomic force microscopy. Analyst 142:3800–3804
Ma C, Liu HY, Tian T, Song XR, Yu JH, Yan M (2016) A simple and rapid detection assay for peptides based on the specific recognition of aptamer and signal amplification of hybridization chain reaction. Biosens Bioelectron 83:15–18
He Y, Lin Y, Hong HW, Pang DW (2012) A graphene oxide-based fluorescent aptasensor for the turn-on detection of epithelial tumor marker mucin 1. Nanoscale 4:2054–2059
Hu R, Wen W, Wang QL, Xiong HY, Zhang XH, Gu HS, Wang SF (2014) Novel electrochemical aptamer biosensor based on an enzyme–gold nanoparticle dual label for the ultrasensitive detection of epithelial tumour marker MUC1. Biosens Bioelectron 53:384–389
Liu CY, Liu X, Qin Y, Deng CY, Xiang J (2016) A simple regenerable electrochemical aptasensor for the parallel and continuous detection of biomarkers. RSC Adv 6:58569–58476
Deng CY, Pi XM, Qian P, Chen XQ, Wu WM, Xiang J (2017) High-performance ratiometric electrochemical method based on the combination of signal probe and inner reference probe in one hairpin-structured DNA. Anal Chem 89:966–973
Jiang XY, Wang HJ, Wang HJ, Zhuo Y, Yuan R, Chai YQ (2017) Electrochemiluminescence biosensor based on 3-D DNA nanomachine signal probe powered by protein-aptamer binding com- plex for ultrasensitive mucin 1 detection. Anal Chem 89:4280–4286
Guo QJ, Li XZ, Shen CC, Zhang SB, Qi HZ, Li T, Yang MH (2015) Electrochemical immunoassay for the protein biomarker mucin 1 and for MCF-7 cancer cells based on signal enhancement by silver nanoclusters. Microchim Acta 182:1483–1489
Yazdanparast S, Benvidi A, Banaei M, Nikukar H, Tezerjani MD, Azimzadeh M (2018) Dual-aptamer based electrochemical sandwich biosensor for MCF-7 human breast cancer cells using silver nanoparticle labels and a poly(glutamic acid)/MWNT nanocomposite. Microchim Acta 185:405
Ma N, Jiang WT, Li T, Zhang ZQ, Qi HZ, Yang MH (2015) Fluorescence aggregation assay for the protein biomarker mucin 1 using carbon dot-labeled antibodies and aptamers. Microchim Acta 182:443–447
Jr MB, Moronne M, Gin P, Weiss S, Alivisatos AP (1998) Semiconductor nanocrystals as fluorescent biological labels. Science 281:2013–2016
Peng ZA, Peng XG (2001) Formation of high-quality CdTe, CdSe, and CdS nanocrystals using CdO as precursor. J Am Chem Soc 123:183–184
Liu ZY, Tang A, Wang M, Yang CH, Teng F (2015) Heating-up synthesis of cadimum-free and color-tunable quaternary and five-component Cu–In–Zn–S-based semiconductor nanocrystals. J Mater Chem C 3:10114–10120
Mao GB, Cai Q, Wang FB, Luo CL, Ji XH, He ZK (2017) One-step synthesis of Rox-DNA functionalized CdZnTeS quantum dots for the visual detection of hydrogen peroxide and blood Gl- ucose. Anal Chem 89:11628–11635
Mao GB, Liu C, Du MY, Zhang YW, Ji XH, He ZK (2018) One-pot synthesis of the stable CdZnTeS quantum dots for the rapid and sensitive detection of copper-activated enzyme. Talanta 185:123–131
Zhao D, Chan WH, He ZK, Qiu T (2009) Quantum dot-ruthenium complex dyads: recognition of double-Strand DNA through dual-color fluorescence detection. Anal Chem 81:3537–3543
Xiang X, Chen L, Zhuang QG, Ji XH, He ZK (2012) Real-time luminescence-based colorimetric determination of double-strand DNA in droplet on demand. Biosens Bioelectron 32:43–49
Liu YF, Luo M, Yan J, Xiang X, Ji XH, Zhou GH, He ZK (2013) An ultrasensitive biosensor for DNA detection based on hybridization chain reaction coupled with the efficient quenching of a ruthenium complex to CdTe quantum dots. Chem Commun 49:7424–7426
Zhang Z, Xiang X, Huang FH, Zheng MM, Xia XY, Han L (2018) Mercury ion-mediated “molecular beacon” integrating with hybridization chain reaction: application to fluorescence turn-on detection of glutathione by using quantum dots and Ru complex. Sensors Actuators B Chem 273:159–166
Liu SW, Xu NH, Tan CY, Fang W, Tan Y (2018) A sensitive colorimetric aptasensor based on trivalent peroxidasemimic DNAzyme and magnetic nanoparticles. Anal Chim Acta 1018:86–93
Huang J, Wu YR, Chen Y, Zhu Z, Yang XH, Yang CYJ (2011) Pyrene-excimer probes based on the hybridization chain reaction for the detection of nucleic acids in complex biological fluids. Angew Chem Int Ed 50:401–404
Ling LS, He ZK, Song GW, Yuan D, Zeng YE (2000) A novel method for determination of DNA by use of molecular ‘light switch’ complex of Ru(bipy)2(dppx)2+. Anal Chim Acta 403:209–217
Zhao D, Fang Y, Wang HY, He ZK (2011) Synthesis and characterization of high-quality water-soluble CdTe: Zn2+ quantum dots capped by N-acetyl-L-cysteine via hydrothermal method. J Mater Chem 21:13365–13370
Ferreira CSM, Matthews CS, Missailidis S (2006) DNA aptamers that bind to MUC1 tumor marker: design and characterization of MUC1-binding single-stranded DNA aptamers. Tumour Biol 27:289–301
Yu WW, Qu LH, Guo WZ, Peng XG (2003) Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem Mater 15:2854–2860
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21675119) and National Major Science and Technology Projects (2018ZX10301405).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 122 kb)
Rights and permissions
About this article
Cite this article
Li, Z., Mao, G., Du, M. et al. A fluorometric turn-on aptasensor for mucin 1 based on signal amplification via a hybridization chain reaction and the interaction between a luminescent ruthenium(II) complex and CdZnTeS quantum dots. Microchim Acta 186, 233 (2019). https://doi.org/10.1007/s00604-019-3347-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3347-3