Abstract
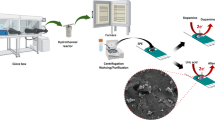
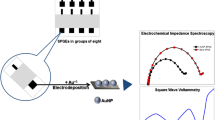
The authors describe a disposable non-enzymatic sensor for ascorbic acid (AA) that was obtained by modifying a screen printed electrode (SPE) with Cu(OH)2 nanorods (NRs). The NRs were synthesized by a wet chemical process which involves sequential addition of NH3 and NaOH to CuSO4 solution. NR formation was confirmed by thermogravimetric, spectroscopic, microscopic, and diffraction studies. The Cu(OH)2 NRs were mixed with carbon ink and printed onto an SPE. Electrochemical detection of AA was carried out at pH 7.4, at a typical voltage as low as 0 mV versus saturated calomel electrode with a scan rate of 100 mV/s, and is assumed to involve the chemical reduction of Cu(II) by AA followed by electrochemical oxidation of Cu(I). The sensor has a linear response in the 0.0125 to 10 mΜ AA concentration range. Response to AA is free from interference by urea, glucose, uric acid, dopamine, metal ions such as Fe2+, Zn2+ and Ni2+, NaCl, KCl and ethanol. It was applied to the determination of AA in a vitamin C tablet and in urine.

A disposable non-enzymatic sensor for ascorbic acid was fabricated using Cu(OH)2 nanorods. The assay is based on the chemical reduction of Cu(II) by AA followed by an electrochemical oxidation of Cu(I). The sensor electrode showed excellent linearity (0.0125–10 mM), sensitivity (268 μA/mM/cm2) and selectivity.




Similar content being viewed by others
References
Bijur GN, Ariza ME, Hitchcock CL, Williams MV (1997) Antimutagenic and promutagenic activity of ascorbic acid during oxidative stress. Environ Mol Mutagen 30(3):339–345
Ambrosi A, Morrin A, Smyth MR, Killard AJ (2008) The application of conducting polymer nanoparticle electrodes to the sensing of ascorbic acid. Anal Chim Acta 609(1):37–43
Farrington AM, Jagota N, Slater JM (1994) Simple solid wire microdisc electrodes for the determination of vitamin C in fruit juices. Analyst 119(2):233–238
Satheesh Babu T, Suneesh P, Ramachandran T, Nair B (2010) Gold nanoparticles modified titania nanotube arrays for amperometric determination of ascorbic acid. Anal Lett 43(18):2809–2822
Prasad KS, Muthuraman G, Zen J-M (2008) The role of oxygen functionalities and edge plane sites on screen-printed carbon electrodes for simultaneous determination of dopamine, uric acid and ascorbic acid. Electrochem Commun 10(4):559–563
Chen W, Lin X, Huang L, Luo H (2005) Electrochemical characterization of polymerized cresol red film modified glassy carbon electrode and separation of electrocatalytic responses for ascorbic acid and dopamine oxidation. Microchim Acta 151(1–2):101–107
Zhang Y, Cai Y, Su S (2006) Determination of dopamine in the presence of ascorbic acid by poly (styrene sulfonic acid) sodium salt/single-wall carbon nanotube film modified glassy carbon electrode. Anal Biochem 350(2):285–291
Kit-Anan W, Olarnwanich A, Sriprachuabwong C, Karuwan C, Tuantranont A, Wisitsoraat A, Srituravanich W, Pimpin A (2012) Disposable paper-based electrochemical sensor utilizing inkjet-printed Polyaniline modified screen-printed carbon electrode for Ascorbic acid detection. J Electroanal Chem 685:72–78
Babu TS, Varadarajan D, Murugan G, Ramachandran T, Nair BG (2012) Gold nanoparticle–polypyrrole composite modified TiO2 nanotube array electrode for the amperometric sensing of ascorbic acid. J Appl Electrochem 42(6):427–434
Yu AM, Zhang HL, Chen HY (1997) Fabrication of a polyglycine chemically modified electrode and its electrocatalytic oxidation to ascorbic acid. Electroanalysis 9(10):788–790
Wu J, Suls J, Sansen W (2000) Amperometric determination of ascorbic acid on screen-printing ruthenium dioxide electrode. Electrochem Commun 2(2):90–93
Zare H, Nasirizadeh N, Ardakani MM (2005) Electrochemical properties of a tetrabromo-p-benzoquinone modified carbon paste electrode. Application to the simultaneous determination of ascorbic acid, dopamine and uric acid. J Electroanal Chem 577(1):25–33
Roy PR, Okajima T, Ohsaka T (2003) Simultaneous electroanalysis of dopamine and ascorbic acid using poly (N, N-dimethylaniline)-modified electrodes. Bioelectrochemistry 59(1):11–19
Aguilar R, Dávila M, Elizalde M, Mattusch J, Wennrich R (2004) Capability of a carbon–polyvinylchloride composite electrode for the detection of dopamine, ascorbic acid and uric acid. Electrochim Acta 49(6):851–859
da Silva RP, Lima AWO, Serrano SH (2008) Simultaneous voltammetric detection of ascorbic acid, dopamine and uric acid using a pyrolytic graphite electrode modified into dopamine solution. Anal Chim Acta 612(1):89–98
Li S-M, Wang Y-S, Hsiao S-T, Liao W-H, Lin C-W, Yang S-Y, Tien H-W, Ma C-CM HC-C (2015) Fabrication of a silver nanowire-reduced graphene oxide-based electrochemical biosensor and its enhanced sensitivity in the simultaneous determination of ascorbic acid, dopamine, and uric acid. J Mater Chem C 3(36):9444–9453
Ruan H, Liu B, Li H (2015) Controlled synthesis of graphene–Gd (OH) 3 nanocomposites and their application for detection of ascorbic acid. RSC Adv 5(27):21242–21248
Chen L-X, Zheng J-N, Wang A-J, Wu L-J, Chen J-R, Feng J-J (2015) Facile synthesis of porous bimetallic alloyed PdAg nanoflowers supported on reduced graphene oxide for simultaneous detection of ascorbic acid, dopamine, and uric acid. Analyst 140(9):3183–3192
Thiagarajan S, Chen S-M (2007) Preparation and characterization of PtAu hybrid film modified electrodes and their use in simultaneous determination of dopamine, ascorbic acid and uric acid. Talanta 74(2):212–222
Zhao D, Fan D, Wang J, Xu C (2015) Hierarchical nanoporous platinum-copper alloy for simultaneous electrochemical determination of ascorbic acid, dopamine, and uric acid. Microchim Acta 182(7–8):1345–1352
Selvaraju T, Ramaraj R (2007) Simultaneous detection of ascorbic acid, uric acid and homovanillic acid at copper modified electrode. Electrochim Acta 52(9):2998–3005
Xi L, Ren D, Luo J, Zhu Y (2010) Electrochemical analysis of ascorbic acid using copper nanoparticles/polyaniline modified glassy carbon electrode. J Electroanal Chem 650(1):127–134
Dursun Z, Nişli G (2004) Voltammetric behavior of copper (I) oxide modified carbon paste electrode in the presence of cysteine and ascorbic acid. Talanta 63(4):873–878
Freire RS, Kubota LT (2002) Electrochemical behavior of the bis (2, 2′-bipyridyl) copper (ii) complex immobilized on a self-assembled monolayer modified electrode for l-ascorbic acid detection. Analyst 127(11):1502–1506
Pei L, Cai Z, Xie Y, Pei Y, Fan C, Fu D (2012) Electrochemical behaviors of ascorbic acid at CuGeO3/polyaniline nanowire modified glassy carbon electrode. J Electrochem Soc 159(10):G107–G111
Pei L, Xie Y, Cai Z, Yang Y, Pei Y, Fan C, Fu D (2012) Electrochemical Behavior of Ascorbic Acid at Copper Germanate Nanowire Modified Electrode. J Electrochem Soc 159(3):K55–K60
Ma Y, Zhao M, Cai B, Wang W, Ye Z, Huang J (2014) 3D graphene foams decorated by CuO nanoflowers for ultrasensitive ascorbic acid detection. Biosens Bioelectron 59:384–388
Rohani T, Taher MA (2009) A new method for electrocatalytic oxidation of ascorbic acid at the Cu (II) zeolite-modified electrode. Talanta 78(3):743–747
Xia C, Ning W (2011) A novel bio-electrochemical ascorbic acid sensor modified with Cu4 (OH) 6SO4 nanorods. Analyst 136(2):288–292
Teixeira MF, Ramos LA, Fatibello-Filho O, Cavalheiro ÉT (2003) Carbon paste electrode modified with copper (II) phosphate immobilized in a polyester resin for voltammetric determination of L-ascorbic acid in pharmaceutical formulations. Anal Bioanal Chem 376(2):214–219
Pei L, Lin N, Wei T, Liu H, Yu H (2015) Formation of copper vanadate nanobelts and their electrochemical behaviors for the determination of ascorbic acid. J Mater Chem A 3(6):2690–2700
Gurav K, Patil U, Shin S, Agawane G, Suryawanshi M, Pawar S, Patil P, Lokhande C, Kim J (2013) Room temperature chemical synthesis of Cu (OH) 2 thin films for supercapacitor application. J Alloys Compd 573:27–31
Lu C, Qi L, Yang J, Zhang D, Wu N, Ma J (2004) Simple template-free solution route for the controlled synthesis of Cu (OH) 2 and CuO nanostructures. J Phys Chem B 108(46):17825–17831
Wang W, Lan C, Li Y, Hong K, Wang G (2002) A simple wet chemical route for large-scale synthesis of Cu (OH) 2 nanowires. Chem Phys Lett 366(3):220–223
Li C, Yin Y, Hou H, Fan N, Yuan F, Shi Y, Meng Q (2010) Preparation and characterization of Cu (OH) 2 and CuO nanowires by the coupling route of microemulsion with homogenous precipitation. Solid State Commun 150(13):585–589
Acknowledgements
Authors greatly acknowledge the Department of Biotechnology (DBT), Government of India for the financial support under the project (Project No BT/PR4076/MED/32/221/2011) and services rendered by PSGTechs COE Indutech, PSG College of Technology, Neelambur. Jeethu Raveendran express her sincere thanks to Council of Scientific and Industrial Research (CSIR) for the financial support under CSIR-SRF Direct scheme (Sanction No. 09/1034(0003)/2 K15-EMR-I).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 1431 kb)
Rights and permissions
About this article
Cite this article
Raveendran, J., Krishnan, R.G., Nair, B.G. et al. Voltammetric determination of ascorbic acid by using a disposable screen printed electrode modified with Cu(OH)2 nanorods. Microchim Acta 184, 3573–3579 (2017). https://doi.org/10.1007/s00604-017-2391-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2391-0