Abstract
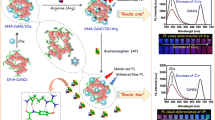
The authors report on the design of a new Förster resonance energy transfer (FRET) based ratiometric nanoprobe for the determination of arginine. The method is based on the inhibition of the efficiency of FRET in assemblies formed between CdTe quantum dots capped with mercaptopropionic acid (QD-MPA) acting as energy donor, and the dye Cresyl Violet (CV) that acts as an energy acceptor at pH 8. Addition of arginine causes a displacement of the CV by arginine on the surface of the QD/MPA. Hence, the FRET between QD/MPA and CV is interrupted and fluorescence emission of the donor (QD/MPA) is restored. Arginine selectively binds to the QD/MPA via electrostatic and hydrogen bonding interactions between guanidinium and carboxylate. Under optimum conditions, the ratio of the fluorescence emissions peaking at 575 and 620 nm (under 400 nm excitation) is linear in the 1 to 30 μM arginine concentration range, and the detection limit is 0.51 μM. The nanoprobe displays good selectivity over 14 other amino acids, many metal ions, glucose, and ascorbic, tartaric and citric acids. The fluorescent nanoprobe was successfully applied to the determination of arginine in pure and spiked real samples and gave good recoveries. Its good selectivity, sensitivity, low-cost and rapidity make the QD-dye assembly a suitable nanoprobe for the quantitation of arginine.

Schematic of a FRET ratiometric nanoprobe for arginine. It is based on quantum dots acting as energy donors and Cresyl Violet acting as energy acceptor. The FRET process is interrupted by the addition of arginine which selectively interacts with carboxy groups via a guanidinium-carboxylate salt bridge.





Similar content being viewed by others
References
Medintz I, Hildebrandt N (2014) FRET-Förster resonance energy transfer: from theory to applications. Wiley-VCH Verlag GmbH & Co. KGaA, Germany, pp 475–583
Algar WR, Tavares AJ, Krull UJ (2010) Beyond labels: a review of the application of quantum dots as integrated components of assays, bioprobes, and biosensors utilizing optical transduction. Anal Chim Acta 673:1–25
Kim JY, Voznyy O, Zhitomirsky D, Sargent EH (2013) 25th anniversary article: colloidal quantum dot materials and devices: a quarter-century of advances. Adv Mater 25:4986–5010
Algar WR, Susumu K, Delehanty JB, Medintz IL (2011) Semiconductor quantum dots in bioanalysis: crossing the valley of death. Anal Chem 83:8826–8837
Chen G, Song F, Xiong X, Peng X (2013) Fluorescent nanosensors based on fluorescence resonance energy transfer (FRET). Ind Eng Chem Res 52:11228–11245
Kim GB, Kim YP (2012) Analysis of protease activity using quantum dots and resonance energy transfer. Theranostics 2:127–138
Dezhurov SV, Volkova IY, Wakstein MS (2011) FRET-based biosensor for oleic acid in nanomolar range with quantum dots as an energy donor. Bioconjug Chem 22:338–345
Algar WR, Kim H, Medintz IL, Hildebrandt N (2014) Emerging non-traditional Förster resonance energy transfer configurations with semiconductor quantum dots: investigations and applications. Coord Chem Rev 263-264:65–85
Ge S, Lu J, Yan M, Yu F, Yu J, Sun X (2011) Fluorescence resonance energy transfer sensor between quantum dot donors and neutral red acceptors and its detection of BSA in micelles. Dyes Pigments 91:304–308
Sha J, Tong C, Zhang H, Feng L, Liu B, Lü C (2015) CdTe QDs functionalized mesoporous silica nanoparticles loaded with conjugated polymers: a facile sensing platform for cupric (II) ion detection in water through FRET. Dyes Pigments 113:102–109
Mobarraz M, Ganjali MR, Chaichi MJ, Norouzi P (2012) Functionalized ZnS quantum dots as luminescent probes for detection of amino acids. Spectrochim Acta-Part A Mol Biomol Spectrosc 96:801–804
Li H, Liang X, Feng L, Liu Y, Wang H (2008) A simple and fast method for arginine determination in grape juice. Food Drug Anal 16:53–58
De Orduña RM (2001) Quantitative determination of L-arginine by enzymatic end-point analysis. J Agric Food Chem 49:549–552
De Orduña RM, Liu SQ, Patchett ML, Pilone GJ (2000) Ethyl carbamate precursor citrulline formation from arginine degradation by malolactic wine lactic acid bacteria. FEMS Microbiol Lett 183:31–35
Sheliakina M, Arkhypova V, Soldatkin O, Saiapina O, Akata B, Dzyadevych S (2014) Urease-based ISFET biosensor for arginine determination. Talanta 121:18–23
Wei YK, Yang J (2007) Evanescent wave infrared chemical sensor possessing a sulfonated sensing phase for the selective detection of arginine in biological fluids. Talanta 71:2007–2014
He L, So VL, Xin JH (2014) A new rhodamine-thiourea/Al3+ complex sensor for the fast visual detection of arginine in aqueous media. Sensors Actuators B Chem 192:496–502
Huang LF, Guo FQ, Liang YZ, Hu QN, Cheng BM (2003) Rapid simultaneous determination of arginine and methylated arginines in human urine by high-performance liquid chromatography–mass spectrometry. Anal Chim Acta 487:145–153
Poon C-Y, Li Q, Zhang J, Li Z, Dong C, Lee AW-M, Chan W-H, Li H-W (2016) FRET-based modified grapheme quantum dots for direct trypsin quantification in urine. Anal Chim Acta 917:64–70
Wu L, Guo Q-S, Liu Y-Q, Sun Q-J (2015) Fluorescence resonance energy transfer-based Ratiometric fluorescent probe for detection of Zn2+ using a dual-emission silica-coated quantum dots mixture. Anal Chem 87:5318–5323
Shanehsaz M, Mohsenifar A, Hasannia S, Pirooznia N, Samaei Y, Shamsipur M (2013) Detection of helicobacter pylori with a nanobiosensor based on fluorescence resonance energy transfer using CdTe quantum dots. Microchim Acta 180:195–202
Mahdi M, Mansour B, Afshin M (2016) Competitive immunoassay for Ochratoxin a based on FRET from quantum dot-labeled antibody to rhodamine-coated magnetic silica nanoparticles. Microchim Acta 183:3093–3099
Wang Y, Ma T, Ma S, Liu Y, Tian Y, Wang R, Wang J (2017) Fluorometric determination of the antibiotic kanamycin by aptamer-induced FRET quenching and recovery between MoS2 nanosheets and carbon dots. Microchim Acta 184:203–210
Zhou R, Wei KY, Zhao JS, Jiang JB (2011) Alternative chiral thiols for preparation of chiral CdS quantum dots covered immediately by achiral thiols. Chem Commun 47:6362–6364
Oluwatobi O, Daramola OA, Ncapayi V (2014) A facile green synthesis of type II water soluble CdTe/CdS core shell nanoparticles. Mater Lett 133:9–13
Yu WW, Qu LH, Guo WZ, Peng XG (2003) Experimental determination of the extinction coefficient of CdTe, CdSe and CdS nanocrystals. Chem Mater 15:2854–2860
Tirado-Guízar A, Pina-Luis G, Paraguay-Delgado F, Ramírez-Herrera D (2014) Size-dependent enhanced energy transfer from tryptophan to CdSe/mercaptopropionic acid quantum dots: a new fluorescence resonance energy transfer nanosensor. Sci Adv Mater 6:492–499
Zhang X, Ju H, Wang J (2008) Electrochemical sensors. Biosensors and their Biomedical Applications. Elsevier, USA, p 158
Lakowicz JR (2006) Principles of fluorescence spectroscopy. Springer, New York, p 678
Gómez-Alonso S, Hermosín-Gutiérrez I, García-Romero E (2007) Simultaneous HPLC analysis of biogenic amines, amino acids, and ammonium ion as aminoenone derivatives in wine and beer samples. J Agric Food Chem 55:608–613
Fiechter G, Mayer HK (2011) UPLC analysis of free amino acids in wines: profiling of on-lees aged wines. J Chromatogr B 879:1361–1366
Mandrioli R, Morganti E, Mercolini L, Kenndler E, Raggi MA (2011) Fast analysis of amino acids in wine by capillary electrophoresis with laser induced fluorescence detection. Electrophoresis 32:2809–2815
Rawat KA, Kailasa SK (2014) Visual detection of arginine, histidine and lysine using quercetin-functionalized gold nanoparticles. Microchim Acta 181:1917–1929
Bogner M, Ludewig U (2007) Visualization of arginine influx into plant cells using a specific FRET-sensor. J Fluoresc 17:350–360
Liang X, Sun J, Chen S, Fan Y (2013) Determination L-arginine from grape juice with Sakaguchi reaction. Adv Mater Res 662:301–304
Pu W, Zhao H, Huang C, Wu L, Xu D (2013) Visual detection of arginine based on the unique guanidine group-induced aggregation of gold nanoparticles. Anal Chim Acta 764:78–83
Kutlán D, Molnár-Perl I (2003) New aspects of the simultaneous analysis of amino acids and amines as their o-phthaldialdehyde derivatives by high-performance liquid chromatography analysis of wine, beer and vinegar. J Chromatogr A 987:311–322
Lozanov V, Petrov S, Mitev S (2004) Simultaneous analysis of amino acid and biogenic polyamines by high-performance liquid chromatography after pre-column derivatization with N-(9-fluorenylmethoxycarbonyloxy) succinimide. J Chromatogr A 1025:201–208
Acknowledgements
The authors gratefully acknowledge support from Consejo Nacional de Ciencia y Tecnología (CONACyT), México (Project 167139), Tecnológico Nacional de México (TecNM) (Project 5623.15-P) and Supramolecular Chemistry Network (CONACyT, Project 271884). D. Ramírez-Herrera thank CONACyT for a Doctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 1204 kb)
Rights and permissions
About this article
Cite this article
Ramírez-Herrera, D.E., Tirado-Guízar, A., Paraguay-Delgado, F. et al. Ratiometric arginine assay based on FRET between CdTe quantum dots and Cresyl violet. Microchim Acta 184, 1997–2005 (2017). https://doi.org/10.1007/s00604-017-2205-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2205-4