Abstract
The authors describe the synthesis of a nanocomposite consisting of Fe3O4 nanoparticles, polypyrrole and graphene oxide (Fe3O4/PPy/GO), and its application to voltammetric sensing of hydrazine. The nanocomposite can be synthesized by combining chemical oxidative polymerization and co-precipitation. Fe(III) ion is employed as both the oxidant for pyrrole and as a precursor of Fe3O4. The nanocomposite was characterized by transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDX), fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS). TEM observations revealed that large numbers of Fe3O4 are homogeneously and densely distributed. The Fe3O4/PPy/GO composite was placed in a glassy carbon electrode, and the resulting sensor, best operated at around 0.2 V (vs. SCE) exhibited excellent response to dissolved hydrazine over the 5.0 μM to 1.3 mM concentration range, a sensitivity of 449.7 μA mM−1 cm−2 and a low detection limit of 1.4 μM (at an S/N ratio of 3).

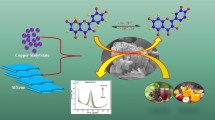
Fe3O4/polypyrrole/graphene oxide nanocomposites were synthesized through one-pot process and then the nanocomposites were used for fabricating a nonenzymatic sensor for hydrazine.






Similar content being viewed by others
References
Jagota NK, Chetram AJ, Nair JB (1998) Determination of trace levels of hydrazine in the penultimate intermediate of a novel anti-infective agent. J Pharm Biomed Anal 16:1083–1087
Cantillo D, Moghaddam MM, Kappe CO (2013) Hydrazine-mediated reduction of nitro and azide functionalities catalyzed by highly active and reusable magnetic iron oxide nanocrystals. J Org Chem 78:4530–4542
Vieira R, Pham-Huu C, Keller N (2002) New carbon nanofiber/graphite felt composite for use as a catalyst support for hydrazine catalytic decomposition. Chem Commun 9:954–955
Ameen S, Akhtar MS, Shin HS (2012) Hydrazine chemical sensing by modified electrode based on in situ electrochemically synthesized polyaniline/graphene composite thin film. Sensors Actuators B Chem 173:177–183
Umar A, Kansal SK, Mehta SK (2013) Highly-sensitive and selective detection of hydrazine at gold electrode modified with PEG-coated CdS nanoparticles. Sensors Actuators B Chem 188:372–377
Gionfriddo E, Naccarato A, Sindona G, Tagarelli A (2014) Determination of hydrazine in drinking water: development and multivariate optimization of a rapid and simple solid phase microextraction-gas chromatography-triple quadrupole mass spectrometry protocol. Anal Chim Acta 835:37–45
Ho TD, Yehl PM, Chetwyn NP (2014) Determination of trace level genotoxic impurities in small molecule drug substances using conventional headspace gas chromatography with contemporary ionic liquid diluents and electron capture detection. J Chromatogr A 1361:217–228
Lee KK, Loh PY, Sow CH (2013) CoOOH nanosheet electrodes: simple fabrication for sensitive electrochemical sensing of hydrogen peroxide and hydrazine. Biosens Bioelectron 39:255–260
Liu Y, Li BB, Wei W (2013) A simple and high-performance hydrazine sensor based on graphene nano platelets supported metal nanoparticles. Adv Mater Res 704:246–251
Koçak S, Aslışen B (2014) Hydrazine oxidation at gold nanoparticles and poly (bromocresol purple) carbon nanotube modified glassy carbon electrode. Sensors Actuators B Chem 196:610–618
Rosca V, Koper MTM (2008) Electrocatalytic oxidation of hydrazine on platinum electrodes in alkaline solutions. Electrochim Acta 53:5199–5205
Lin H, Yang J, Liu J (2013) Properties of Pd nanoparticles-embedded polyaniline multilayer film and its electrocatalytic activity for hydrazine oxidation. Electrochim Acta 90:382–392
Wang Y, Yang X, Bai J (2013) High sensitivity hydrogen peroxide and hydrazine sensor based on silver nanocubes with rich {100} facets as an enhanced electrochemical sensing platform. Biosens Bioelectron 43:180–185
Rahman MM, Khan A, Marwani HM, Asiri AM (2016) Hydrazine sensor based on silver nanoparticle-decorated polyaniline tungstophosphate nanocomposite for use in environmental remediation. Microchim Acta 183:1787–1796
Zhang J, Gao WB, Dou ML, Wang F, Liu JJ, Li ZL, Ji J (2015) Nanorod-constructed porous Co3O4 nanowires: highly sensitive sensors for the detection of hydrazine. Analyst 5:1686–1692
Khan SB, Faisal M, Rahman MM (2013) Highly sensitive and stable phenyl hydrazine chemical sensors based on CuO flower shapes and hollow spheres. New J Chem 37:1098–1104
Shukla S, Chaudhary S, Umar A (2014) Tungsten oxide (WO3) nanoparticles as scaffold for the fabrication of hydrazine chemical sensor. Sensors Actuators B Chem 196:231–237
Cabana J, Monconduit L, Larcher D (2010) Beyond intercalation-based li-ion batteries: the state of the art and challenges of electrode materials reacting through conversion reactions. Adv Mater 22:E170–E192
Okada T, Takeda Y, Watanabe N (2014) Chemically stable magnetic nanoparticles for metal adsorption and solid acid catalysis in aqueous media. J Mater Chem A 2:5751–5758
Zhang Z, Zhu H, Wang X (2011) Sensitive electrochemical sensor for hydrogen peroxide using Fe3O4 magnetic nanoparticles as a mimic for peroxidase. Microchim Acta 174:183–189
Yu C, Gou L, Zhou X (2011) Chitosan-Fe3O4 nanocomposite based electrochemical sensors for the determination of bisphenol A. Electrochim Acta 56:9056–9063
He Y, Sheng Q, Liu B (2012) Synthesis of three-dimensional network Fe3O4 at gas/liquid interface and its sensing application. Electrochim Acta 66:82–87
Huang X, Qi X, Boey F (2012) Graphene-based composites. Chem Soc Rev 41:666–686
Salavagione HJ, Díez-Pascual AM, Lázaro E (2014) Chemical sensors based on polymer composites with carbon nanotubes and graphene: the role of the polymer. J Mater Chem A 2:14289–14328
Yao W, Ni T, Chen S (2014) Graphene/Fe3O4@ polypyrrole nanocomposites as a synergistic adsorbent for Cr (VI) ion removal. Combust Sci Technol 99:15–22
Liu P, Huang Y, Zhang X (2014) Synthesis and excellent microwave absorption properties of graphene/polypyrrole composites with Fe3O4 particles prepared via a co-precipitation method. Mater Lett 129:35–38
Ren S, Ma S, Yang Y (2015) Hydrothermal synthesis of Fe2O3/polypyrrole/graphene oxide composites as highly efficient electrocatalysts for oxygen reduction reaction in alkaline electrolyte. Electrochim Acta 178:179–189
Perera SD, Mariano RG, Vu K (2012) Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activity. ACS Catal 2:949–956
Lu X, Mao H, Zhang W (2009) Fabrication of core-shell Fe3O4/polypyrrole and hollow polypyrrole microspheres. Polym Compos 30:847–854
Su J, Cao M, Ren L (2011) Fe3O4–graphene nanocomposites with improved lithium storage and magnetism properties. J Phys Chem C 115:14469–14477
Wu J, Zhou T, Wang Q (2016) Morphology and chemical composition dependent synthesis and electrochemical properties of MnO2-based nanostructures for efficient hydrazine detection. Sensors Actuators B Chem 224:878–884
Aziz MA, Kawde AN (2013) Gold nanoparticle-modified graphite pencil electrode for the high-sensitivity detection of hydrazine. Talanta 115:214–221
Haghighi B, Hamidi H, Bozorgzadeh S (2010) Sensitive and selective determination of hydrazine using glassy carbon electrode modified with Pd nanoparticles decorated multiwalled carbon nanotubes. Anal Bioanal Chem 398:1411–1416
Harraz FA, Ismail AA, Al-Sayari SA (2016) Highly sensitive amperometric hydrazine sensor based on novel α-Fe2O3/crosslinked polyaniline nanocomposite modified glassy carbon electrode. Sensors Actuators B Chem 234:573–582
Salimi A, Miranzadeh L, Hallaj R (2008) Amperometric and voltammetric detection of hydrazine using glassy carbon electrodes modified with carbon nanotubes and catechol derivatives. Talanta 75:147–156
Ding Y, Wang Y, Zhang L (2011) Preparation of TiO2-Pt hybrid nanofibers and their application for sensitive hydrazine detection. Nano 3:1149–1157
Zhu J, Chauhan DS, Shan D, Wu XY, Zhang GY, Zhang XJ (2014) Ultrasensitive determination of hydrazine using a glassy carbon electrode modified with pyrocatechol violet electrodeposited on single walled carbon nanotubes. Microchim Acta 181:813–820
He YP, Zheng JB, Sheng QL (2012) Cobalt nanoparticles as sacrificial templates for the electrodeposition of palladium nanomaterials in an ionic liquid, and its application to electrochemical sensing of hydrazine. Microchim Acta 177:479–484
Acknowledgements
The authors gratefully acknowledge the financial support of this project by the National Science Fund of China (No. 21275116, No. 21575113), Specialized Research Fund for the Doctoral Program of Higher Education (No. 20126101110013), the Scientific Research Foundation of Shaanxi Provincial Key Laboratory (14JS094, 15JS100, 16JS099).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 7913 kb)
Rights and permissions
About this article
Cite this article
Yang, Z., Sheng, Q., Zhang, S. et al. One-pot synthesis of Fe3O4/polypyrrole/graphene oxide nanocomposites for electrochemical sensing of hydrazine. Microchim Acta 184, 2219–2226 (2017). https://doi.org/10.1007/s00604-017-2197-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2197-0