Abstract
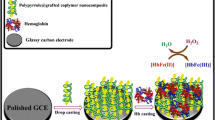
Core-shell Au@Ag nanorods (Ag@GNRs) were synthesized and utilized to construct a voltammetric biosensor for trichloroacetic acid (TCA). The biosensor was prepared by immobilizing hemoglobin (Hb) on a glassy carbon electrode (GCE) that was modified with the Ag@GNRs. Cyclic voltammetry revealed a pair of symmetric redox peaks, indicating that direct electron transfer occurs at the Hb on the Ag@GNR-film. The electron transfer rate constant is as high as 2.32 s−1. The good electrocatalytic capability and large surface area of the Ag@GNR-film is beneficial in terms of electron transfer between Hb and the underlying electrode. The modified GCE, best operated at −0.4 V (vs. SCE), exhibits electrocatalytic activity toward TCA in the 0.16 μM to 1.7 μM concentration range, with a 0.12 μM detection limit (at an S/N ratio of 3).

Core-shell Au@Ag nanorods (Ag@GNRs) were synthesized and used to immobilize hemoglobin to construct an effective biosensor for trichloroacetic acid.




Similar content being viewed by others
References
Mai Z, Zhao X, Dai Z, Zou X (2010) Direct electrochemistry of hemoglobin adsorbed on self-assembled monolayers with different head groups or chain length. Talanta 81:167–175
Saadati S, Salimia A, Hallaj R, Rostami A (2014) Direct electron transfer and electrocatalytic properties of immobilized hemoglobin onto glassy carbon electrode modified with ionic-liquid/titanium-nitride nanoparticles: application to nitrite detection. Sensor Actuat B-Chem 191:625–633
Palanisamy S, Wang YT, Chen SM, Thirumalraj B, Lou BS (2016) Direct electrochemistry of immobilized hemoglobin and sensing of bromate at a glassy carbon electrode modified with graphene and β-cyclodextrin. Microchim Acta 183:1953–1961
Wu YF, Xue P, Kang YJ, Hui KM (2013) Highly specific and ultrasensitive graphene-enhanced electrochemical detection of low-abundance tumor cells using silica nanoparticles coated with antibody-conjugated quantum dots. Anal Chem 85:3166–3173
Huang QT, Zhang HQ, Hu SR, Li FM, Weng W, Chen JH, Wang QX, He YS, Zhang WX, Bao XX (2014) A sensitive and reliable dopamine biosensor was developed based on the Au@carbon dots-chitosan composite film. Biosens Bioelectron 52:277–280
Zang S, Liu YJ, Lin MH, Kang JL, Sun YM, Lei HT (2013a) A dual amplified electrochemical immunosensor for ofloxacin: polypyrrole film-Au nanocluster as the matrix and multi-enzyme-antibody functionalized gold nanorod as the label. Electrochim Acta 90:246–253
Navaei A, Saini H, Christenson W, Sullivan RT, Ros R, Nikkhah M (2016) Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater 41:133–146
Khlebtsov BN, Khanadeev VA, Ye J, Sukhorukov GB, Khlebtsov NG (2014) Overgrowth of gold nanorods by using a binary surfactant mixture. Langmuir 30:1696–1703
Komathi S, Gopalan AI, Kim SK, Anand GS, Lee KP (2013) Fabrication of horseradish peroxidase immobilized poly(n-[3-(trimethoxy silyl)propyl]aniline) gold nanorods film modified electrode and electrochemical hydrogen peroxide sensing. Electrochim Acta 92:71–78
Bai W, Huang H, Li Y, Zhang H, Liang B, Guo R (2014a) Direct preparation of well-dispersed graphene/gold nanorod composites and their application in electrochemical sensors for determination of ractopamine. Electrochim Acta 117:322–328
Shakoori Z, Salimian S, Kharrazi S, Adabi M, Saber R (2015) Electrochemical DNA biosensor based on gold nanorods for detecting hepatitis B virus. Anal Bioanal Chem 407:455–461
Azimzadeh M, Rahaie M, Nasirizadeh N, Ashtari K, Naderi-Manesh H (2016) An electrochemical nanobiosensor for plasma miRNA-155, based on graphene oxide and gold nanorod, for early detection of breast cancer. Biosens Bioelectron 77:99–106
Yang XJ, Wang YH, Liu YW, Jiang XE (2013) A sensitive hydrogen peroxide and glucose biosensor based on gold/silver core–shell nanorods. Electrochim Acta 108:39–44
Zhang Y, Lu F, Yan Z, Wu D, Ma H, Du B (2015a) Electrochemiluminescence immunosensing strategy based on the use of Au@Ag nanorods as a peroxidase mimic and NH4CoPO4 as a supercapacitive supporter: application to the determination of carcinoembryonic antigen. Microchim Acta 182:1–9
Tang LJ, Li S, Han F, Liu LQ, Xu LG, Ma W, Kuang H, Li AK, Wang LB, Xu CL (2015) SERS-active Au@Ag nanorod dimers for ultrasensitive dopamine detection. Biosens Bioelectron 71:7–12
Sun JD, Ji J, Sun YQ, Abdalhai MH, Zhang YZ, Sun XL (2015) DNA biosensor-based on fluorescence detection of E. coli O157:H7 by Au@Ag nanorods. Biosens Bioelectron 70:239–245
Zhang HF, Ning DL, Ma L, Zheng JB (2016) Silver deposition directed by self-assembled gold nanorods for amplified electrochemical immunoassay. Anal Chim Acta 902:82–88
Bull RJ, Sanchez IM, Nelson MA, Larson JL, Lansing AJ (1990) Liver tumor induction in B6C3F1 mice by dichloroacetate and trichloroacetate. Toxicology 63:341–359
Dojlido J, Zbieć E, Świetlik R (1999) Formation of the haloacetic acids during ozonation and chlorination of water in Warsaw waterworks (Poland). Water Res 33:3111–3118
Ferreira AMC, Laespada MEF, Pavon JLP, Cordero BM (2013) In situ derivatization coupled to microextraction by packed sorbent and gas chromatography for the automated determination of haloacetic acids in chlorinated water. J Chromatogr A 1318:35–42
Prieto-Blanco MC, Alpendurada MF, López-Mahía P, Muniategui-Lorenzo S, Prada-Rodríguez D, Machado S, Gonçalves C (2012) Improving methodological aspects of the analysis of five regulated haloacetic acids in water samples by solid-phase extraction, ion-pair liquid chromatography and electrospray tandem mass spectrometry. Talanta 94:90–98
Boysen G (2009) Liquid chromatography electrospray ionization tandem mass spectrometry analysis method for simultaneous detection of trichloroacetic acid, dichloroacetic acid, S-(1,2-dichlorovinyl)glutathione and S-(1,2-dichlorovinyl)-L-cysteine. Toxicology 262:230–238
Dai H, Xu H, Wu X, Lin Y, Wei M, Chen G (2010) Electrochemical behavior of thionine at titanate nanotubes-based modified electrode: a sensing platform for the detection of trichloroacetic acid. Talanta 81:1461–1466
Zhan T, Wang X, Zhang Y, Song Y, Liu X, Xu J (2015) Direct electrochemistry and electrocatalysis of hemoglobin immobilized in layered double hydroxides modified with amino functionalized ionic liquid through coprecipitation technique. Sensor Actuat B-Chem 220:1232–1240
Ding Y, Wang Y, Lei Y (2010) Direct electrochemistry and electrocatalysis of novel single-walled carbon nanotubes–hemoglobin composite microbelts—towards the development of sensitive and mediator-free biosensor. Biosens Bioelectron 26:390–397
Sun W, Sun ZL, Zhang LQ, Qi XW, Li GJ, Wu J, Wang M (2013a) Application of Fe3O4 mesoporous sphere modified carbon ionic liquid electrode as electrochemical hemoglobin biosensor. Colloid Surface B 101:177–182
Sun W, Guo YQ, Ju XM, Zhang YY, Wang XZ, Sun ZF (2013b) Direct electrochemistry of hemoglobin on graphene and titanium dioxide nanorods composite modified electrode and its electrocatalysis. Biosens Bioelectron 42:207–213
Sun W, Guo YQ, Lu YP, Hu AH, Shi F, Li TT, Sun ZF (2013c) Electrochemical biosensor based on graphene, Mg2Al layered double hydroxide and hemoglobin composite. Electrochim Acta 91:130–136
Liu Y, Han T, Chen C, Bao N, Yu CM, Gu HY (2011) A novel platform of hemoglobin on core-shell structurally Fe3O4@Au nanoparticles and its direct electrochemistry. Electrochim Acta 56:3238–3247
Ye XC, Zheng C, Chen J, Gao YZ, Murray CB (2013) Using binary surfactant mixtures to simultaneously improve the dimensional tunability and monodispersity in the seeded growth of gold nanorods. Nano Lett 13:765–771
Casas J, Venkataramasubramani M, Wang YY, Tang L (2013) Replacement of cetyltrimethylammoniumbromide bilayer on gold nanorod by alkanethiol crosslinker for enhanced plasmon resonance sensitivity. Biosens Bioelectron 49:525–530
Meng J, Ji YL, Liu J, Cheng XL, Guo H, Zhang WQ, Wu XC, Xu HY (2014) Using gold nanorods core/silver shell nanostructures as model material to probe biodistribution and toxic effects of silver nanoparticles in mice. Nanotoxicology 8:686–696
Wang XK, Chen L, Chen LX (2014) Colorimetric determination of copper ions based on the catalytic leaching of silver from the shell of silver-coated gold nanorods. Microchim Acta 181:105–110
Yu C, Zhou X, Gu H (2010) Immobilization, direct electrochemistry and electrocatalysis of hemoglobin on colloidal silver nanoparticles-chitosan film. Electrochim Acta 55:8738–8743
Feng Q, Liu K, Fu J, Zhang Y, Zheng Z, Wang C (2012) Direct electrochemistry of hemoglobin based on nano-composite film of gold nanopaticles and poly (diallyldimethylammonium chloride) functionalized graphene. Electrochim Acta 60:304–308
Fu Q, Liu HL, Wu Z, Liu A, Yao C, Li X (2015) Rough surface Au@Ag core–shell nanoparticles to fabricating high sensitivity SERS immunochromatographic sensors. J Nanobiotechnol 13:1–9
Bai T, Sun J, Che R, Xu L, Yin C, Guo Z (2014b) Controllable preparation of core-shell Au-Ag nanoshuttles with improved refractive index sensitivity and SERS activity. ASC Appl Mater Inter 6:3331–3340
Jayabal S, Ramaraj R (2014) Bimetallic Au/Ag nanorods embedded in functionalized silicate sol–gel matrix as an efficient catalyst for nitrobenzene reduction. Appl Catal A-Gen 470:369–375
Zhang S, Han L, Hou C, Li C, Lang Q, Han L (2015b) Novel glucose sensor with Au@Ag heterogeneous nanorods based on electrocatalytic reduction of hydrogen peroxide at negative potential. J Electroanal Chem 742:84–89
Royo B, Sosna M, Asensio AC, Moran JF, Ferapontova EE (2013) Direct electrochemistry and environmental sensing of rice hemoglobin immobilized at graphite electrodes. J Electroanal Chem 704:67–74
Laviron E (1979a) The use of linear potential sweep voltammetry and of a.C. Voltammetry for the study of the surface electrochemical reaction of strongly adsorbed systems and of redox modified electrodes. J Electroanal Chem 100:263–270
Laviron E (1979b) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101:19–28
Liu Y, Jiang QY, Lu SY, Zhang Y (2009) Immobilization of hemoglobin on the gold colloid modified pretreated glassy carbon electrode for preparing a novel hydrogen peroxide biosensor. Appl Biochem Biotech 152:418–427
Bond AM (1980) Modern Polarographic methods in analytical chemistry. Marcel Dekker, New York, pp p29–p30
Yamazaki I, Araiso T, Hayashi Y, Yamada H, Makino R (1978) Analysis of acid-base properties of peroxidase and myoglobin. Adv Biophys 11:249–281
Herzog G, Kam V, Arrigan DWM (2008) Electrochemical behaviour of haemoglobin at the liquid/liquid interface. Electrochim Acta 53:7204–7209
Shie JW, Yogeswaran U, Chen SM (2009) Haemoglobin immobilized on nafion modified multi-walled carbon nanotubes for O2, H2O2 and CCl3COOH sensors. Talanta 78:896–902
Zhan T, Wang X, Li X, Song Y, Hou W (2016) Hemoglobin immobilized in exfoliated Co2Al LDH-graphene nanocomposite film: direct electrochemistry and electrocatalysis toward trichloroacetic acid. Sensor Actuat B-Chem 228:101–108
Zhu Z, Qu L, Niu Q, Zeng Y, Sun W, Huang X (2011) Urchinlike MnO2, nanoparticles for the direct electrochemistry of hemoglobin with carbon ionic liquid electrode. Biosens Bioelectron 26:2119–2124
Kamin RA, Wilson GS (1980) Rotating ring-disk enzyme electrode for biocatalysis kinetic studies and characterization of the immobilized enzyme layer. Anal Chem 52:1198–1205
Sun W, Dong L, Deng Y, Yu J, Wang W, Zhu Q (2014) Application of N-doped graphene modified carbon ionic liquid electrode for direct electrochemistry of hemoglobin. Mat Sci Eng C-Mater 39:86–91
Sun W, Zhang YY, Wang XZ, Ju XM, Wang D, Wu J, Sun ZF (2012) Electrodeposited graphene and silver nanoparticles modified electrode for direct electrochemistry and electrocatalysis of hemoglobin. Electroanalysis 24:1973–1979
Ruan CX, Li TT, Niu QJ, Lu M, Lou J, Gao WM, Sun W (2012) Electrochemical myoglobin biosensor based on graphene-ionic liquid-chitosan bionanocomposites: direct electrochemistry and electrocatalysis. Electrochim Acta 64:183–189
Kurd M, Salimi A, Hallaj R (2013) Highly sensitive amperometric sensor for micromolar detection of trichloroacetic acid based on multiwalled carbon nanotubes and Fe(II)-phtalocyanine modified glassy carbon electrode. Mat Sci Eng C-Mater 33:1720–1726
Sun W, Cao L, Deng Y, Gong S, Shi F, Li G (2013d) Direct electrochemistry with enhanced electrocatalytic activity of hemoglobin in hybrid modified electrodes composed of graphene and multi-walled carbon nanotubes. Anal Chim Acta 781:41–47
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21375066 and 21475070), the Natural Science Foundation of Jiangsu Province (BK20151267), the Qing Lan Project of Jiangsu Province, the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the Application Research Item of Nantong City (MS12015046).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 150 kb)
Rights and permissions
About this article
Cite this article
Qian, D., Li, W., Chen, F. et al. Voltammetric sensor for trichloroacetic acid using a glassy carbon electrode modified with Au@Ag nanorods and hemoglobin. Microchim Acta 184, 1977–1985 (2017). https://doi.org/10.1007/s00604-017-2175-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2175-6