Abstract
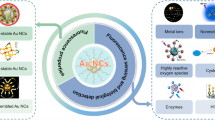
We describe a ratiometric colorimetric method for the determination of coenzyme A (CoA) by using gold nanoparticles (AuNPs) and bis-uranyl-bis-sulfosalophen (BUBSS) as optical probes. BUBSS is a binuclear uranyl complex and formed through the chelating reaction of two uranyl ions with bis-sulfosalophen. CoA is captured by the AuNPs via the thiol group and this leads to the formation of CoA-AuNPs. In a second step, BUBSS binds two CoA-AuNPs through a coordination reaction between the uranyl ions in BUBSS and the phosphate groups in CoA-AuNPs. This causes the CoA-AuNPs to aggregate and results in a color change from wine red to blue. A ratiometric colorimetric assay was established for CoA based on the ratiometric measurement of absorbance changes at 650 and 525 nm. Their ratio is linearly related to the concentration of CoA in the 0 to 1.2 μmol⋅L-1 range, with a 6 nmol⋅L-1 detection limit under optimal conditions. The method was successfully applied to the determination of CoA in spiked liver samples with recoveries between 99.4 and 102.6 %.

Gold nanoparticles (AuNPs) capture Coenzyme A (CoA) firstly and then bind bis-uranyl-bis-sulfosalophen (BUBSS). This causes their aggregation and results in a color change. A ratiometric colorimetric assay was established for CoA based on measuring absorbances at 630 and 525 nm.





Similar content being viewed by others
References
Davaapil H, Tsuchiya Y, Gout I (2014) Signaling functions of coenzyme a and its derivatives in mammalian cells. Biochem Soc Trans 42:1056–1062
Martinez DL, Tsuchiya Y, Gout I (2014) Coenzyme a biosynthetic machinery in mammalian cells. Biochem Soc Trans 42:1112–1117
Allred JB, Guy DG (1969) Determination of coenzyme a and acetyl CoA in tissue extracts. Anal Biochem 29:293–299
McDougal Jr DB, Dargar RV (1979) A spectrophotometric cycling assay for reduced coenzyme a and its esters in small amounts of tissue. Anal Biochem 97:103–115
Marques SM, Esteves da Silva JCG (2008) An optimized luciferase bioluminescent assay for coenzyme a. Anal Bioanal Chem 391:2161–2168
Shibata K, Nakai T, Fukuwatari T (2012) Simultaneous high-performance liquid chromatography determination of coenzyme a, dephospho-coenzyme a, and acetyl-coenzyme a in normal and pantothenic acid-deficient rats. Anal Biochem 430:151–155
Halvorsen O, Skrede S (1980) Separation of coenzyme-a and its precursors by reversed-phase high-performance liquid-chromatography. Anal Biochem 107:103–108
Li J, Ge X, Jiang C (2007) Spectrofluorimetric determination of trace amounts of coenzyme a using terbium ion-ciprofloxacin complex probe in the presence of periodic acid. Anal Bioanal Chem 387:2083–2089
Vallejos S, Estevez P, Ibeas S, Garcia FC, Serna F, Garcia JM (2012) An organic/inorganic hybrid membrane as a solid "turn-on" fluorescent chemosensor for coenzyme a (CoA), cysteine (Cys), and glutathione (GSH) in aqueous media. Sensors 12:2969–2982
Cheng M, Qiang X, Du C (2013) Fluorescent detection of coenzyme a by analyte-induced aggregation of a cationic conjugated polymer. Chinese Sci Bull 58:1256–1261
Giljanovic J, Prkic A (2010) Determination of coenzyme a (CoASH) in the presence of different thiols by using flow-injection with a UV/vis spectrophotometric detector and potentiometric determination of CoASH using an iodide ISE. Molecules 15:100–113
Liu J, Lu Y (2006) Preparation of aptamer-linked gold nanoparticle purple aggregates for colorimetric sensing of analytes. Nat Protoc 1:246–252
Maity D, Bhatt M, Paul P (2015) Calix[4]arene functionalized gold nanoparticles for colorimetric and bare-eye detection of iodide in aqueous media and periodate aided enhancement in sensitivity. Microchim Acta 182:377–384
Ratnarathorn N, Chailapakul O, Dungchai W (2015) Highly sensitive colorimetric detection of lead using maleic acid functionalized gold nanoparticles. Talanta 132:613–618
Pandya A, Joshi KV, Modi NR, Menon SK (2012) Rapid colorimetric detection of sulfide using calix[4]arene modified gold nanoparticles as a probe. Sens Actuat B 168:54–61
Akhond M, Absalan G, Ershadifar H (2015) Highly sensitive colorimetric determination of amoxicillin in pharmaceutical formulations based on induced aggregation of gold nanoparticles. Spectrochim Acta A 143:223–229
Kumar N, Seth R, Kumar H (2014) Colorimetric detection of melamine in milk by citrate-stabilized gold nanoparticles. Anal Biochem 456:43–49
Giannoulis KM, Giokas DL, Tsogas GZ, Vlessidis AG (2014) Ligand-free gold nanoparticles as colorimetric probes for the non-destructive determination of total dithiocarbamate pesticides after solid phase extraction. Talanta 119:276–283
Storhoff JJ, Elghanian R, Mucic RC, Mirkin CA, Letsinger RL (1998) One-pot colorimetric differentiation of polynucleotides with single base imperfections using gold nanoparticle probes. J Am Chem Soc 120:1959–1964
Thavanathan J, Huang NM, Thong KL (2014) Colorimetric detection of DNA hybridization based on a dual platform of gold nanoparticles and graphene oxide. Biosen Bioelectron 55:91–98
Verdoold R, Gill R, Ungureanu F, Molenaar R, Kooyman RPH (2011) Femtomolar DNA detection by parallel colorimetric darkfield microscopy of functionalized gold nanoparticles. Biosens Bioelectron 27:77–81
Choi I, Yang YI, Jeong E, Kim K, Hong S, Kang T, Yi J (2012) Colorimetric tracking of protein structural evolution based on the distance-dependent light scattering of embedded gold nanoparticles. Chem Commun 48:2286–2288
Carey JR, Suslick KS, Hulkower KI, Imlay JA, Imlay KRC, Ingison CK, Ponder JB, Sen A, Wittrig AE (2011) Rapid identification of bacteria with a disposable colorimetric sensing array. J Am Chem Soc 133:7571–7576
Templeton AC, Chen S, Gross SM, Murray RW (1999) Water-soluble, isolable gold clusters protected by tiopronin and coenzyme a monolayers. Langmuir 15:66–76
Rudkevich DM, Verboom W, Brzozka Z, Palys MJ, Stauthamer WPRV, Van Hummel GJ, Franken SM, Harkema S, Engbersen JFJ, Reinhoudt DN (1994) Functionalized UO2 salenes: neutral receptors for anions. J Am Chem Soc 116:4341–4351
Sessler JL, Melfi PJ, Pantos GD (2006) Uranium complexes of multidentate N-donor ligands. Coordin Chem Rev 250:816–843
Antonisse MMG, Snellink-Ruël BHM, Engbersen JFJ, Reinhoudt DN (1998) H2PO- 4-selective CHEMFETs with uranyl salophene receptors. Sensors Actuators B Chem 47:9–12
Kim J, Kang DM, Shin SC, Choi MY, Kim J, Lee SS, Kim JS (2008) Functional polyterthiophene-appended uranyl-salophen complex: electropolymerization and ion-selective response for monohydrogen phosphate. Anal Chim Acta 614:85–92
Wojciechowski K, Wróblewski W, Brzózka Z (2003) Anion buffering in the internal electrolyte resulting in extended durability of phosphate-selective electrodes. Anal Chem 75:3270–3273
Wróblewski W, Wojciechowski K, Dybko A, Brzózka Z, Egberink RJM, Snellink-Ruël BHM, Reinhoudt DN (2000) Uranyl salophenes as ionophores for phosphate-selective electrodes. Sensors Actuators B Chem 68:313–318
Wygladacz K, Qin Y, Wroblewski W, Bakker E (2008) Phosphate-selective fluorescent sensing microspheres based on uranyl salophene ionophores. Anal Chim Acta 614:77–84
He Y, Liao L, Xu C, Wu R, Li S, Yang Y (2015) Determination of ATP by resonance light scattering using a binuclear uranyl complex and aptamer modified gold nanoparticles as optical probes. Microchim Acta 182:419–426
Liu G, Chen J, Che P, Ma Y (2003) Separation and quantitation of short-chain coenzyme a’s in biological samples by capillary electrophoresis. Anal Chem 75:78–82
Acknowledgments
The authors thank the National Natural Science Foundation of China (NSFC Nos. 11275091, 11475079) for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 111 kb)
Rights and permissions
About this article
Cite this article
Wu, R., Liao, L., Li, S. et al. Ratiometric colorimetric determination of coenzyme A using gold nanoparticles and a binuclear uranyl complex as optical probes. Microchim Acta 183, 715–721 (2016). https://doi.org/10.1007/s00604-015-1716-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1716-0