Abstract
Purpose
In Japan, the administration of S-1 following D2 gastrectomy is a standard treatment for stage II/III gastric cancer (GC). However, the survival of stage IIIB/IIIC GC remains unsatisfactory. To improve this, we conducted a multicenter phase II study to evaluate the safety and efficacy of a neoadjuvant S-1 and oxaliplatin regimen (SOX) followed by surgery targeted at stage III GC.
Methods
Oxaliplatin was administered intravenously (130 mg/m2) on day 1, and S-1 was administered orally (40 mg/m2, twice a day) for 14 days followed by a seven-day rest period. After three cycles of therapy, D2 gastrectomy was performed.
Results
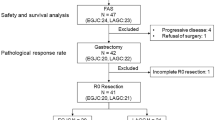
A total of 14 patients were enrolled and completed the protocol treatment. Grade 3/4 toxicities included thrombocytopenia (21.4 %), anorexia (14.3 %), and diarrhea (7.1 %). Seven patients (50 %) underwent total gastrectomy, and seven patients underwent distal gastrectomy. Grade 3/4 surgical complications included pancreatic fistula (21.4 %) and lung infection (7.1 %). The pathological response rate was 85.7 %.
Conclusion
Although our data are limited and preliminary, neoadjuvant SOX followed by surgery can be performed safely with a high pathological response rate in patients with resectable advanced GC. Further investigation of this neoadjuvant approach is warranted.
Similar content being viewed by others
References
Okuyama T, Korenaga D, Edagawa A, Itoh S, Oki E, Kawanaka H, et al. Prognostic effect of oral anti-cancer drugs as adjuvant chemotherapy for 2 years after gastric cancer surgery. Surg Today. 2012;42:734–40.
Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-years outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–93.
Cascinu S, Labianca R, Barone C, Santoro A, Camaghi C, Cassano A, et al. Adjuvant treatment of high-risk, radically resected gastric cancer patients with 5-fluorouracil, leucovorin, cisplatin, and epidoxorubicin in a randomized controlled trial. J Natl Cancer Inst. 2007;99:601–7.
Di Costanzo F, Gasperoni S, Manzione L, Bisagni G, Labianca R, Bravi S, et al. Adjuvant chemotherapy in completely resected gastric cancer: a randomized phase III trial conducted by GOIRC. J Natl Cancer Inst. 2008;100:388–98.
Kodera Y, Ishiyama A, Yoshikawa T, Kinoshita T, Ito S, Yokoyama H, et al. A feasibility study of postoperative chemotherapy with S-1 and cisplatin (CDDP) for gastric carcinoma (CCOG0703). Gastric Cancer. 2010;13:197–203.
Yoshikawa T, Rino Y, Yukawa N, Oshima T, Tsuburaya A, Masuda M. Neoadjuvant chemotherapy for gastric cancer in Japan: a standing position by comparing with adjuvant chemotherapy. Surg Today. 2014;44:11–21.
Kodera Y, Kobayashi D, Tanaka C, Fujiwara M. Gastric adenocarcinoma with para-aortic lymph node metastasis: a borderline resectable cancer? Surg Today. 2015;45:1082–90.
Aizawa M, Nashimoto A, Yabusaki H, Nakagawa S, Matsuki A, Homma K, Kawasaki T. The clinical significance of potentially curative resection for gastric cancer following the clearance of free cancer cells in the peritoneal cavity by induction chemotherapy. Surg Today. 2015;45:611–7.
Okazaki SE, Nakajima T, Hashimoto J, Yamamoto S, Takahari D, Kato K, et al. A feasibility study of outpatient chemotherapy with S-1 + cisplatin in patients with advanced gastric cancer. Gastric Cancer. 2013;16:41–7.
Kidani Y, Inagaki K, Tsukagoshi S. Examination of antitumor activities of platinum complexes of 1, 2-diaminocyclohexane isomers and their related complexes. Gann. 1976;67:921–2.
Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46.
Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, et al. Phase III study comparing oxaliplatin plus S-1 in chemotherapy-naïve patients advanced gastric cancer. Ann Oncol. 2015;26:141–8.
Association Japanese Gastric Cancer. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113–23.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma. 14th ed. Tokyo: Kanehara Inc; 2010.
Yamada Y, Takahari D, Matsumoto H, Baba H, Nakamura M, Yoshida K, et al. Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2013;14:1278–86.
Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–21.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Veide CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M, et al. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy—Japan Clinical Oncology Group Study 9501. J Clin Oncol. 2004;22:2767–73.
Iwasaki Y, Sasako M, Yamamoto S, Nakamura K, Sano T, Katai H, et al. Phase II study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). J Surg Oncol. 2013;107:741–5.
Nakamura K, Kuwata T, Shimoda T, Mizusawa J, Katayama H, Kushima T, et al. Determination of the optimal cutoff percentage of residual tumors to define the pathological response rate for gastric cancer treated with preoperative therapy (JCOG1004-A). Gastric Cancer. 2015;18:597–604.
Park I, Ryu MH, Choi YH, Kang HJ, Yook JH, Park YS, et al. A phase II study of neoadjuvant docetaxel, oxaliplatin, and S-1 (DOS) chemotherapy followed by surgery and adjuvant S-1 chemotherapy in potentially resectable gastric or gastroesophageal junction adenocarcinoma. Cancer Chemother Pharmacol. 2013;72:815–23.
Acknowledgments
We thank Mr. Yushi Nagai, Ms. Michiyo Tada, and Ms. Emiko Usami for their assistance with data management. This study was supported by the National Cancer Center Research and Development Fund (Grant no. 23-A-2). Oxaliplatin was provided by Yakult Honsha Co., Ltd. Y.Y. received lecture fees from Taiho.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The other authors have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Honma, Y., Yamada, Y., Terazawa, T. et al. Feasibility of neoadjuvant S-1 and oxaliplatin followed by surgery for resectable advanced gastric adenocarcinoma. Surg Today 46, 1076–1082 (2016). https://doi.org/10.1007/s00595-015-1276-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-015-1276-2