Abstract
Aims
To test potential efficacy of liraglutide, a GLP-1 receptor agonist, in subjects with type 1 diabetes (T1DM).
Methods
We have recruited nine T1DM patients (age 40.1 ± 6.4 years, duration of diabetes 19.2 ± 8.8 years, BMI 24.3 ± 3.5 kg/m2, HbA1c 8.2 ± 1.0 %–66 ± 11 mmol/mol, daily insulin dose: 0.6 ± 0.1 IU/kg) on continuous subcutaneous insulin therapy with undetectable C-peptide. In addition to existing treatment was administered in single-blind (a) therapy subcutaneously with 0.1 ml of saline solution for 3 days and (b) 0.1 ml of liraglutide (0.6 mg/day) for a further 3 days with daily glucose excursions recorded by continuous glucose monitoring.
Results
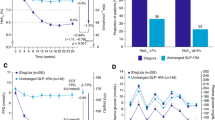
Adding liraglutide resulted in a significant reduction in mean blood glucose (138 ± 29 vs. 163 ± 29 mg/dl, p < 0.0001) and standard deviation (42 ± 9 vs. 60 ± 15 mg/dl, p < 0.0001). The area under the curve (AUC) for blood glucose >140 mg/dl was also significantly reduced (22.2 ± 16.4 vs. 41.1 ± 19.7 mg/dl h, p < 0.05) with no difference in AUC for blood glucose <70 mg/dl (liraglutide 0.7 ± 0.9 mg/dl h; placebo: 0.8 ± 1.4 mg/dl h, p = NS). Finally, adding liraglutide reduced daily insulin requirement (37.5 ± 17.2 vs. 42.9 ± 22.4 UI/day, p < 0.01).
Conclusions
Short-term treatment with liraglutide, in T1DM, reduces average blood glucose, blood glucose variability and daily insulin requirement without increasing risk of hypoglycemia.

Similar content being viewed by others
Abbreviations
- T1DM:
-
Type 1 diabetes mellitus
- AUC:
-
Area under the curve
- GLP-1 RA:
-
Glucagon-like peptide 1 receptor agonist
- CSII:
-
Continuous subcutaneous insulin therapy
References
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR (2015) Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 38:140–149
Campbell JE, Drucker DJ (2013) Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 17:819–837
Bruttomesso D, Fongher C, Silvestri B, Barberio S, Marescotti MC, Iori E, Valerio A, Crazzolara D, Pianta A, Tiengo A, Del Prato S (2001) Combination of continuous subcutaneous infusion of insulin and octreotide in Type 1 diabetic patients. Diab Res Clin Pract 51:97–105
Kielgast U, Holst JJ, Madsbad S (2009) Treatment of type 1 diabetic patients with glucagon-like peptide-1 (GLP-1) and GLP-1R agonists. Curr Diabetes Rev 5:266–275
Kuhaydiya ND, Malik R, Bellini NJ et al (2013) Liraglutide as additional treatment to insulin in obese patients with type 1 diabetes mellitus. EndocrPract 19:963–967
Harrison LB, Mora PF, Clark GO, Lingvay I (2013) Type 1 diabetes treatment beyond insulin: role of GLP-1 analogs. J Investig Med 61:40–44
Varanasi A et al (2011) Liraglutide as additional treatment for type 1 diabetes. Eur J Endocrinol 165:77–84
Kumar KVH, Shaikh A, Prusty P (2013) Addition of exenatide or sitagliptin to insulin in new onset type 1 diabetes: a randomized, open label study. Diab Res Clin Pract 100:55–58
Rorther KL, Spain LM, Wesley RA et al (2009) Effects of exenatide alone and in combination with daclizumab on beta-cell function in long-standing type 1 diabetes. Diabetes Care 32:2251–2257
Traina AN, Lull ME, Hui AC, Zahorian TM, Lyons-Patterson J (2014) Once-weekly exenatide as adjunct treatment of type 1 diabetes mellitus in patients receiving continuous subcutaneous insulin infusion therapy. Can J Diabets 38:269–272
Kielgast U, Holst JJ, Madsbad S (2011) Antidiabetic actions of endogenous and exogenous GLP-1 in type 1 diabetic patients with and without residual beta-cell function. Diabetes 60:1599–1607
Sarkar G, Alattar M, Brown RJ et al (2014) Exenatide treatment for 6 months improves insulin sensitivity in adults with type 1 diabetes. Diabetes Care 37:666–670
Singh S, Chang HY, Richards TM, Weiner JP, Clark JM, Segal JB (2013) Glucagon like peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med 173:534–539
Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC (2011) Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 141:150–156
Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC (2013) Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 62:2595–2604
Halfdanarson TR, Pannala R (2013) Incretins and risk of neoplasia. BMJ 346:f3750
Faillie JL, Babai S, Crépin S, Bres V, Laroche ML, Le Louet H, Petit P, Montastruc JL, Hillaire-Buys D, French Pharmacovigilance Centers Network (2014) Pancreatitis associated with the use of GLP-1 analogs and DPP-4 inhibitors: a case/non-case study from the French Pharmacovigilance Database. Acta Diabetol 51:491–497
Bonner-Weir S, In’t Veld PA, Weir GC (2014) Reanalysis of sftudy of pancreatic effects of incretin therapy: methodological deficiencies. Diabetes Obes Metab 16:661–666
Harja E, Skyler JS (2013) An analysis of characteristics of subjects examined for incretin effects on pancreatic pathology. Diabetes Technol Ther 15:609–618
Egan AG, Blind E, Dunder K, de Graeff PA, Hummer BT, Bourcier T, Rosebraugh C (2014) Pancreatic safety of incretin - based dugs—FDA and EMA assessment. N Engl J Med 370:794–797
Fiorentino TV, Owston M, Abrahamiam G, La Rosa S, Marando A, Prengo C, Di Cairano ES, Finzi G, Capella C, Sessa F, Casiraghi F, Paez A, Adivi A, Davalli A, Fiorina P, Guardado Mendoza R, Comuzzie AG, Sharp M, DeFronzo RA, Halff G, Dick EJ, Folli F (2015) Chronic continuous exenatide infusion does not cause pancreatic inflammation and ductal hyperplasia in non-human primates. Am J Pathol 185:139–150
Dejgaard TF, Knop FK, Tamow L, Frandsen CS, Hansen TS, Almdal T, Holst JJ, Madsbad S, Andersen HU (2015) Efficacy and safety of the glucagon-like peptide-1 receptor agonist liraglutide added to insulin therapy in poorly regulated patients with type 1 diabetes—a protocol for a randomized, double-blind, placebo-controlled study: the Lira-1 study. BMJ Open 5:e007791
Acknowledgments
IC was supported in part by a fellowship grant from Fondazione Diabete Ricerca-MSD. The study was supported by the PRIN grant 2010 YK7Z5K_006 and grant 2010 JS3PMZ_002.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SDP has received honoraria for advisory work and lectures from Astra Zeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Intarcia, Janssen, Merck Sharpe and Dohme, Novartis, Novo Nordisk, Roche Diagnostics, sanofi aventis and Takeda as well as research support from Bristol-Myers Squibb, Merck Sharpe and Dohme, Novartis and Novo Nordisk. IC, MA, SKP and GD have no conflict to declare.
Human and animal rights disclosure
All procedures followed were in accordance with the Ethical Standards of the responsible Institutional Committee on Human Experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
Informed consent was obtained from all patients from being included in the study.
Additional information
Managed by Massimo Federici.
Rights and permissions
About this article
Cite this article
Crisci, I., Aragona, M., Politi, K.S. et al. GLP-1 receptor agonists in type 1 diabetes: a proof-of-concept approach. Acta Diabetol 52, 1129–1133 (2015). https://doi.org/10.1007/s00592-015-0800-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-015-0800-6