Abstract
Aim
Diabetic patients commonly suffer from disturbances in production and clearance of plasma lipoproteins, known as diabetic dyslipidemia, resulting in an increased risk of coronary heart disease. The study aimed to examine the cause of hypobetalipoproteinemia in two patients with type 1 diabetes.
Methods
The Diabetes Control and Complications Trial (DCCT) is a study demonstrating that intensive blood glucose control delays the onset and progression of type 1 diabetes complications. Hypobetalipoproteinemia was present in two DCCT subjects, IDs 1427 and 1078, whose LDL-C levels were 36 and 28 mg/dL, respectively, and triglyceride levels were 20 and 28 mg/dL, respectively. We performed exome sequencing on genomic DNA from the two patients with hypobetalipoproteinemia.
Results
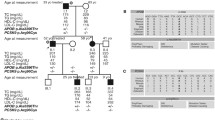
The subjects 1427 and 1078 had heterozygous loss-of-function mutations in the gene apolipoprotein B (ApoB), and these mutations resulted in premature stop codons at amino acid 1333 (ApoB-29) and 3680 (ApoB-81), respectively. Indeed, the plasma ApoB level of subject 1427 (19 mg/dL) was the lowest and that of subject 1078 (26 mg/dL) was the second to the lowest among all the 1,441 DCCT participants. Sequencing genomic DNA of family members showed that probands 1427 and 1078 inherited the mutations from the father and the mother, respectively.
Conclusions
The identification of ApoB loss-of-function mutations in type 1 diabetic patients presents innovative cases to study the interaction between hypobetalipoproteinemia and insulin deficiency.



Similar content being viewed by others
Abbreviations
- ApoB:
-
Apolipoprotein B
- BMI:
-
Body mass index
- DCCT:
-
Diabetes control and complications trial
- EDIC:
-
Epidemiology of diabetes interventions and complications
- FHBL:
-
Familial hypobetalipoproteinemia
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL:
-
Low-density lipoprotein
- LDL-C:
-
Low-density lipoprotein cholesterol
- Lp(a):
-
Lipoprotein (a)
- NIDDK:
-
National Institute of Diabetes and Digestive and Kidney diseases
- SNP:
-
Single nucleotide polymorphism
- TAG:
-
Triacylglycerol
References
Lusis AJ (2000) Atherosclerosis. Nature 407(6801):233–241
Maranhao RC, Carvalho PO, Strunz CC, Pileggi F (2014) Lipoprotein (a): structure, pathophysiology and clinical implications. Arq Bras Cardiol 103(1):76–84
Lisak M, Demarin V, Trkanjec Z, Basic-Kes V (2013) Hypertriglyceridemia as a possible independent risk factor for stroke. Acta Clin Croat 52(4):458–463
Varbo A, Nordestgaard BG (2014) Remnant cholesterol and ischemic heart disease. Curr Opin Lipidol 25(4):266–273
Packard CJ, Shepherd J (1997) Lipoprotein heterogeneity and apolipoprotein B metabolism. Arterioscler Thromb Vasc Biol 17(12):3542–3556
Hussain MM, Shi J, Dreizen P (2003) Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res 44(1):22–32
Fisher EA, Zhou M, Mitchell DM, Wu X, Omura S, Wang H, Goldberg AL, Ginsberg HN (1997) The degradation of apolipoprotein B100 is mediated by the ubiquitin-proteasome pathway and involves heat shock protein 70. J Biol Chem 272(33):20427–20434
Linton MF, Farese RV Jr, Young SG (1993) Familial hypobetalipoproteinemia. J Lipid Res 34(4):521–541
Welty FK (2014) Hypobetalipoproteinemia and abetalipoproteinemia. Curr Opin Lipidol 25(3):161–168
Tarugi P, Averna M (2011) Hypobetalipoproteinemia: genetics, biochemistry, and clinical spectrum. Adv Clin Chem 54:81–107
Whitfield AJ, Barrett PH, van Bockxmeer FM, Burnett JR (2004) Lipid disorders and mutations in the APOB gene. Clin Chem 50(10):1725–1732
Schonfeld G (1995) The hypobetalipoproteinemias. Annu Rev Nutr 15:23–34
Haffner SM (2003) Management of dyslipidemia in adults with diabetes. Diabetes Care 26(Suppl 1):S83–S86
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F (2005) Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 112(17):2735–2752
Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M (1998) Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339(4):229–234
Goldberg IJ (2001) Clinical review 124: diabetic dyslipidemia: causes and consequences. J Clin Endocrinol Metab 86(3):965–971
Mooradian AD (2009) Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab 5(3):150–159
Adiels M, Boren J, Caslake MJ, Stewart P, Soro A, Westerbacka J, Wennberg B, Olofsson SO, Packard C, Taskinen MR (2005) Overproduction of VLDL1 driven by hyperglycemia is a dominant feature of diabetic dyslipidemia. Arterioscler Thromb Vasc Biol 25(8):1697–1703
The DCCT Research Group (1991) Epidemiology of severe hypoglycemia in the diabetes control and complications trial. The DCCT Research Group. Am J Med 90(4):450–459
The DCCT Research Group (1998) Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. The diabetes control and complications trial research group. Ann Intern Med 128(7):517–523
Nathan DM, Bayless M, Cleary P, Genuth S, Gubitosi-Klug R, Lachin JM, Lorenzi G, Zinman B (2013) Diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: advances and contributions. Diabetes 62(12):3976–3986
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25(14):1754–1760
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25(16):2078–2079
The DCCT Research Group (2003) Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 290(16):2159–2167
The DCCT Research Group (1999) Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 22(1):99–111
de Boer IH, Kestenbaum B, Rue TC, Steffes MW, Cleary PA, Molitch ME, Lachin JM, Weiss NS, Brunzell JD (2008) Insulin therapy, hyperglycemia, and hypertension in type 1 diabetes mellitus. Arch Intern Med 168(17):1867–1873
Murdoch SJ, Boright AP, Paterson AD, Zinman B, Steffes M, Cleary P, Edwards K, Marcovina SS, Purnell JQ, Brunzell JD (2007) LDL composition in E2/2 subjects and LDL distribution by Apo E genotype in type 1 diabetes. Atherosclerosis 192(1):138–147
Sibley SD, Hokanson JE, Steffes MW, Purnell JQ, Marcovina SM, Cleary PA, Brunzell JD (1999) Increased small dense LDL and intermediate-density lipoprotein with albuminuria in type 1 diabetes. Diabetes Care 22(7):1165–1170
Shendure J, Mitra RD, Varma C, Church GM (2004) Advanced sequencing technologies: methods and goals. Nat Rev Genet 5(5):335–344
Ginsburg GS, Willard HF (2009) Genomic and personalized medicine: foundations and applications. Transl Res 154(6):277–287
Cirulli ET, Goldstein DB (2010) Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nat Rev Genet 11(6):415–425
Ng SB, Buckingham KJ, Lee C, Bigham AW, Tabor HK, Dent KM, Huff CD, Shannon PT, Jabs EW, Nickerson DA, Shendure J, Bamshad MJ (2010) Exome sequencing identifies the cause of a mendelian disorder. Nat Genet 42(1):30–35
Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA, Shendure J (2011) Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet 12(11):745–755
Gilissen C, Hoischen A, Brunner HG, Veltman JA (2012) Disease gene identification strategies for exome sequencing. Eur J Hum Genet 20(5):490–497
Ballestri S, Lonardo A, Losi L, Pellegrini E, Bertolotti M, Loria P (2009) Do diabetes and obesity promote hepatic fibrosis in familial heterozygous hypobetalipoproteinemia? Intern Emerg Med 4(1):71–73
Ohashi K, Ishibashi S, Yamamoto M, Osuga J, Yazaki Y, Yukawa S, Yamada N (1998) A truncated species of apolipoprotein B (B-38.7) in a patient with homozygous hypobetalipoproteinemia associated with diabetes mellitus. Arterioscler Thromb Vasc Biol 18(8):1330–1334
Sankatsing RR, Fouchier SW, de Haan S, Hutten BA, de Groot E, Kastelein JJ, Stroes ES (2005) Hepatic and cardiovascular consequences of familial hypobetalipoproteinemia. Arterioscler Thromb Vasc Biol 25(9):1979–1984
Turk U, Basol G, Barutcuoglu B, Sahin F, Habif S, Tarugi P, Bayindir O (2012) A 54-year-old diabetic man with low serum cholesterol. Clin Chem 58(5):826–829
Pulai JI, Latour MA, Kwok PY, Schonfeld G (1998) Diabetes mellitus in a new kindred with familial hypobetalipoproteinemia and an apolipoprotein B truncation (apoB-55). Atherosclerosis 136(2):289–295
Targher G, Arcaro G (2007) Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis 191(2):235–240
Acknowledgments
The current study was supported in part by a fund from Wayne State University to R.Z., NNSF of China (90408028) to C.T.Z. and (31171238) to F.G. and the China National 863 High-Tech Program (2015AA020101) to F.G.
Conflict of interest
Feng Gao, Hao Luo, Zhiyao Fu, Chun-Ting Zhang and Ren Zhang declare that they have no conflict of interest.
Human and Animal Rights disclosure
All procedures followed were in accordance with the ethical standards of the Institutional Review Boards of Wayne State University School of Medicine and of the Detroit Medical Center, and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed consent disclosure
Informed consent was obtained from all participants being included in the study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Managed by Massimo Porta.
Rights and permissions
About this article
Cite this article
Gao, F., Luo, H., Fu, Z. et al. Exome sequencing identifies novel ApoB loss-of-function mutations causing hypobetalipoproteinemia in type 1 diabetes. Acta Diabetol 52, 531–537 (2015). https://doi.org/10.1007/s00592-014-0687-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-014-0687-7