Abstract
Purpose
To identify the factors associated with a correction of the segmental angle (SA) with a total change greater than 10° in each level following minimally invasive oblique lumbar interbody fusion (MIS-OLIF).
Methods
Patients with lumbar spinal stenosis who underwent single- or two-level MIS-OLIF were reviewed. Segments with adequate correction of the SA >10° after MIS-OLIF in immediate postoperative radiograph were categorized as discontinuous segments (D segments), whereas those without such improvement were assigned as continuous segments (C segments). Clinical and radiological parameters were compared, and multivariate logistic regression analysis was performed to identify factors associated with SA correction >10° after MIS-OLIF.
Results
Of 211 segments included, 38 segments (18.0%) were classified as D segments. Compared with C segments, D segments demonstrated a significantly smaller preoperative SA (mean ± standard deviation [SD], − 1.1° ± 6.7° vs. 6.6° ± 6.3°, p < 0.001), larger change of SA (mean ± SD, 13.5° ± 3.4° vs. 3.1° ± 3.9°, p < 0.001), and a higher rate of presence of facet effusion (76.3% vs. 48.6%, p = 0.002). Logistic regression revealed preoperative SA (odds ratio (OR) [95% confidence interval (CI)]:0.733 [0.639–0.840], p < 0.001) and facet effusion (OR [95% CI]:14.054 [1.758–112.377], p = 0.027) as significant predictors for >10° SA correction after MIS-OLIF.
Conclusion
Preoperative kyphotic SA and facet effusion can predict SA correction >10° following MIS-OLIF. For patients with lordotic SA and no preoperative facet effusion, supplemental procedures, such as anterior column release or posterior osteotomy, should be prepared for additional lumbar lordosis correction required for remnant global sagittal imbalance after MIS-OLIF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Minimally invasive oblique lateral interbody fusion (MIS-OLIF) has been reported to have potential advantages in minimally violating the paraspinal muscles and restoring segmental lordosis more efficiently than posterior and transforaminal lumbar interbody fusion (PLIF and TLIF, respectively) [1,2,3,4,5]. In OLIF or extreme lateral interbody fusion (XLIF), because a larger cage than in PLIF and TLIF can be used, optimal correction of decreased lumbar lordosis (LL) is easier. This plays a crucial role in improving clinical outcomes and preventing adjacent segment disease (ASD) [6, 7].
Addressing sagittal imbalance is critical even in short-segment fusion because optimal reduction of pelvic tilt (PT), enhancement of the C7 sagittal vertical axis (SVA), and harmonization of pelvic incidence (PI), and LL can reduce the incidence of postoperative lower back pain, lower extremity pain, and ASD [6,7,8]. Therefore, achieving adequate correction of lordosis at the index surgical level is important for improving clinical outcomes in patients undergoing MIS-OLIF.
Despite the superior corrective potential of OLIF and XLIF versus TLIF and PLIF, typical per-level corrections after OLIF or XLIF have been reported to be < 10° [9]. However, several studies have shown changes in regional LL >10° after XLIF [10,11,12,13]. This discrepancy implies that there is a specific condition in which sufficient correction of the segmental angle (SA) is expected; however, the clinical and radiological factors associated with sufficient correction remain underexplored. Therefore, this study aimed to identify the preoperative or postoperative radiological factors associated with adequate correction of the SA greater than 10° at each surgical level and to establish a predictive formula for SA correction following MIS-OLIF with percutaneous pedicle screw instrumentation.
Materials and methods
Patients
We retrospectively reviewed patients who underwent single- or two-level MIS-OLIF between August 2012 and March 2023. Given the retrospective nature of this study, the Institutional Review Board waived the requirement for informed consent. The inclusion criteria were as follows: (1) undergoing single- or two-level MIS-OLIF with percutaneous pedicle screw instrumentation and (2) preoperative C7 SVA >50 mm. The exclusion criteria were as follows: (1) unavailability of immediate postoperative spine radiographs, (2) concurrent compression fracture at the time of OLIF, and (3) congenital hip dysplasia, congenital stenosis, malignancy, inflammatory disease, or infection. We obtained demographic and clinical data, such as age, sex, body mass index (BMI), preoperative diagnosis, surgical level, fusion level, prior surgery at the index level, height and angle of the inserted intervertebral cage, and perioperative complications from electronic medical records and the picture archiving and communication system.
Surgery
Most patients underwent circumferential MIS-OLIF via the anterior retroperitoneal approach in the lateral decubitus position. A polyether-ether-ketone intervertebral cage filled with demineralized bone matrix was placed after discectomy to enhance the posterior disc space height. Cages with lordotic angles of 6°, 8°, or 12° were implanted in all patients, positioned between the middle and anterior third of the disc space. We aimed to maximize disc height without resorting to anterior column release (ACR) or posterior osteotomy, therefore neither ACR nor posterior osteotomy was not used in the OLIF procedure. Patients were then repositioned prone for posterior percutaneous pedicle screw insertion under intraoperative fluoroscopy. When the patient was in the prone position, we used a chest bar and two posts beneath both anterior superior iliac spines to achieve maximum lumbar sagging. The hip position was nearly extended to maximize LL in the lower segments. Screws were inserted in situ, without the use of any compression or distraction manoeuvers.
Radiological assessment
Two authors, blinded to the clinical data, reviewed preoperative and immediate postoperative standing lumbosacral and whole-spine standing radiographs, along with preoperative axial and sagittal T1- and T2-weighted magnetic resonance (MR) images. Preoperative radiographs were assessed for disc height, spondylolisthesis, angular and translational instability, spondylolysis, and disc vacuum at each surgical level. Instability was defined as a range of motion exceeding 10 degrees or vertebral body translation exceeding 3 mm in disc space when comparing flexion and extension lumbar lateral X-rays. The preoperative and immediate postoperative SAs were examined at each surgical level. Preoperative and immediate postoperative sagittal parameters, such as PI, LL between the L1 and S1 upper endplates, the sacral slope, PT, thoracic kyphosis (TK) between the T5 and T12 endplates, and C7 SVA, were evaluated.
The radiological characteristics of preoperative MR images at the index surgical level were examined. These included the preoperative Pfirrmann grade [14], osteoarthritis grading of the facet joints [15], presence of facet cyst and facet effusion [16], and the Goutallier grade of the paraspinal muscle at the L5–S1 disc level [17]. Central and foraminal stenoses were evaluated using the Schizas and Lee grades, respectively [18, 19].
Definition of D and C segments
Costanzo demonstrated that lateral lumbar interbody fusion typically resulted in average corrections of less than 10° per level, without the need for ACR or posterior osteotomy [9]. Therefore, the segments that exhibited adequate segmental correction >10° after MIS-OLIF without ACR were categorized as discontinuous segments (D segments), whereas those without such improvement were assigned as continuous segments (C segments) (Figs. 1 and 2). The term “discontinuous” refers to the acute interruption in a single, smooth lumbar lordotic curve, contrasting with the “continuous” type, which signifies a smooth curvature transition. This nomenclature is derived from mathematical distinctions between continuous and non-continuous function graphs.
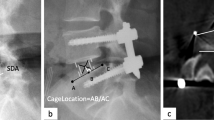
A 66-year old female patient who underwent two-level oblique lumbar interbody fusion with both D and C segment. (a) Preoperative standing lumbar lateral radiograph showing C segment at L3–4 and D segment at L4–5 with acute disruption of smooth lumbar lordotic curve. (b) Preoperative magnetic resonance imaging showing bilateral facet effusion at L4–5 level. (c) Preoperative standing whole-spine lateral radiograph showing global sagittal malalignment. (d) Postoperative standing lumbar lateral radiograph and (e) standing whole-spine lateral radiograph showing restoration of smooth lumbar lordotic curve, resulting in adequate correction of preoperative global sagittal imbalance. D discontinuous, C continuous
A 62-year old female patient who underwent single level oblique lumbar interbody fusion with D segment. (a) Preoperative standing lumbar lateral radiograph showing D segment at L4–5 with spondylolytic spondylolisthesis showing acute disruption of smooth lumbar lordotic curve. (b) Preoperative standing whole-spine lateral radiograph showing global sagittal malalignment. (c) Postoperative standing lumbar lateral radiograph and (d) standing whole-spine lateral radiograph showing restoration of smooth lumbar lordotic curve, resulting in adequate correction of preoperative global sagittal imbalance. D discontinuous
Statistical analysis
Continuous variables showing differences between D and C segments were assessed using Student’s t test or the Kruskal–Wallis test, whereas categorical variables were evaluated using chi-squared, Fisher’s exact, or linear-by-linear association tests. Logistic regression identified preoperative factors predicting SA correction >10° after MIS-OLIF at each index surgical level. To optimize variable selection and reduce the risk of omitting potentially significant factors, variables demonstrating significant associations in the univariate logistic regression (p < 0.20) were included in the multivariate model. Backward elimination was used to calculate the odds ratios (OR) and 95% confidence intervals (CI) for stooping improvement. Receiver operating characteristic (ROC) curves were used to calculate the area under the curve (AUC), gauge sensitivity, and specificity of risk factors in predicting stooping improvement. AUC values of 0.5–0.7, 0.7–0.9, and >0.9 denoted low, moderate, and high discriminatory power, respectively. The relationship between the identified preoperative radiological risk factors, which were continuous variables, and the degree of SA correction was evaluated using Pearson’s correlation coefficient and simple linear regression analysis, resulting in a predictive formula for SA correction. All statistical analyses were performed using IBM SPSS Statistics (version 25.0; IBM Corp., Armonk, NY).
Results
Our study included 211 segments in 148 patients with a mean age (± standard deviation [SD]) of 71.4 ± 8.0 years (Fig. 3). Overall, 35 patients (23.6%) had D segments at the index surgical level. Thirty-eight segments (18.0%) were categorized as D segments, and 173 segments (82.0%) were categorized as C segments. Table 1 displays the demographic and radiological characteristics of patients with and without D segment. No significant intergroup differences were observed in age, sex, BMI, number of surgical levels, or the specific surgical levels. Among all patients, 96 (64.9%) showed an improvement in C7 SVA < 50 mm after surgery. Radiologically, patients with D segment exhibited significantly larger preoperative PI minus LL, PT, and C7 SVA and significantly smaller preoperative LL and TK compared with patients without D segment. There were no significant differences in the postoperative LL, PI minus LL, and C7 SVA between D and C segments after MIS-OLIF. In the OLIF procedure, the mean cage height was 11.5 ± 1.4 mm for the D segment and 11.1 ± 1.7 mm for the C segment, with no significant differences noted between the two groups (p = 0.136). Similarly, the mean cage angle was 8.6 ± 2.8° in the D segment and 7.7 ± 2.5° in the C segment, showing no significant difference between groups (p = 0.069).
Table 2 presents a comparison of the preoperative and postoperative radiographical parameters between D and C segments. D segments demonstrated a significantly larger proportion of decreased disc height (92.1% vs. 74.6%, p = 0.019), significantly smaller preoperative SA (mean ± SD, −1.1° ± 6.7° vs. 6.6° ± 6.3°, p < 0.001), and significantly larger postoperative SA (mean ± SD, 12.4° ± 6.0° vs. 9.7° ± 5.4°, p = 0.006) and change of SA (mean ± SD, 13.5° ± 3.4° vs. 3.1° ± 3.9°, p < 0.001) compared with C segments. Although a higher proportion of patients with D segment displayed spondylolisthesis, spondylolysis, angular instability, and translational instability compared with patients with C segment, these differences were not significant.
Table 3 compares the radiological parameters of preoperative MR images between D and C segments. D segments had a significantly higher proportion of sequestrated discs (35.1% vs. 16.3%, p = 0.009) and facet effusion (76.3% vs. 48.6%, p = 0.002) compared with C segments. No significant differences were noted in the rate of facet joint osteoarthritis or the Schizas and Lee grades.
In the multivariate logistic regression analyses, preoperative SA (OR [95% CI]:0.733 [0.639–0.840], p < 0.001) and the presence of facet effusion (OR [95% CI]:14.054 [1.758–112.377], p = 0.027) were significant predictors for >10° SA correction after MIS-OLIF at each index surgical level. (Table 4, the full version of Table 4 in the Appendix).
ROC curve analysis identified threshold values predicting >10° SA correction after MIS-OLIF at each surgical level. Preoperative SA showed moderate discriminative power (AUC: 0.800, 95% CI: 0.717–0.882, p < 0.001) with a cutoff value of −0.05° (Fig. 4), implying that patients with preoperative kyphotic SA values are likely to achieve SA changes >10° after MIS-OLIF. The combination of facet effusion with preoperative kyphotic SA enhanced this predictability (OR = 10.5; specificity = 0.91; positive predictive value = 0.56). In segments displaying preoperative lordotic SA without facet effusion, the likelihood of failing to achieve an SA change >10° after MIS-OLIF was 95% (negative predictive value = 0.95).
Strong correlations were observed between the preoperative SA and degree of SA correction (r = −0.618, p < 0.001). Predictive formulae for the degree of SA correction were established using preoperative SA and the presence of facet effusion. For segments with facet effusion, the formula was as follows: degree of SA correction = −0.560 × preoperative SA + 8.431 (R2 = 0.478, p < 0.001; Fig. 5a). For segments without facet effusion, the formula was: SA correction = −0.354 × preoperative SA + 5.853 (R2 = 0.226, p < 0.001; Fig. 5b).
Discussion
Our study revealed that the presence of preoperative kyphotic SA and facet effusion can predict >10° SA correction after MIS-OLIF at each surgical index level. These correctable segments were defined as D segments. However, in patients presenting with preoperative lordotic SA without facet effusion, the likelihood of achieving >10° SA correction after MIS-OLIF is limited. Hence, supplemental procedures such as ACR or posterior osteotomy might be required for additional correction of the decreased LL.
We defined segments that showed a >10° SA correction following MIS-OLIF as discontinuous segments (D segments). The term “discontinuous” was used because of the acute disruption of continuity within a single smooth lumbar lordotic curve. Examples of D segments are shown in Figs. 1 and 2, where the entire lumbar curve appears interrupted at the L4–5 level, which is the D segment. This disruption in D segment can be rectified by MIS-OLIF, resulting in a smooth lumbar curve. This concept parallels the disruption of the Shenton line at the hip joint [20].
Patients with D segment showed a more severe preoperative global sagittal imbalance with a larger preoperative PI minus LL and C7 SVA compared with patients without D segment; however, the difference disappeared after MIS-OLIF. Both patient groups, those with and without D segments, demonstrated acceptable postoperative LL, PI minus LL, and C7 SVA (Table 1). Patients with D segment showed a smaller postoperative C7 SVA after MIS-OLIF compared with patients without D segment, although the difference was not significant. These findings suggest that the D segment significantly affects global sagittal imbalance and that correcting the D segment can result in adequate correction of the preoperative global sagittal imbalance. Our results align with those of Huang’s study, indicating that a smaller preoperative PI, PT, or PI–LL predicts sagittal spinal realignment in older patients with LSS after short-segment decompression and fusion surgery [21].
D segments showed more severe preoperative regional loss of lordosis with a significantly smaller preoperative SA compared with C segments. However, after MIS-OLIF, D segments showed significantly larger postoperative SA compared with C segments. Furthermore, the change in SA in D segments after MIS-OLIF was significantly higher, with a mean value of 13.5°, than that in C segments (Table 2). Previous research demonstrating a >10° change in regional LL after XLIF often involved the use of 20° or 30° hyperlordotic cages [10, 13]. Youn et al. also recommended using hyperlordotic angle cages in short-segment lumbar fusion for better correction of LL [22]. However, in our clinical experience, without performing ACR, inserting a cage with an angle significantly larger than the endplate angle did not lead to widening of the disc space in accordance with the cage angle. Instead, a discrepancy between the endplate and cage angles reduced the contact area between them, often resulting in endplate fracture. This phenomenon was particularly pronounced in patients with osteoporosis. Therefore, we primarily used cages with a 6° or 12° lordotic angle without additional ACR or posterior osteotomy to maximize disc space height with intact anterior longitudinal ligament. Thus, our findings represent the inherent correction potential for lordosis in D segments.
In logistic regression, the identification of preoperative factors predicting >10° SA correction after MIS-OLIF revealed that a smaller SA and the presence of facet effusion were significant predictors. This is corroborated by Alahmari and Zhu, who demonstrated that preoperative kyphotic discs acquire more lordosis with interbody cage use [23, 24]. They suggested that kyphotic discs possess greater corrective potential for lordosis, making positive lordotic alterations easier to attain than lordotic discs. Their explanation is also consistent with our results of smaller preoperative SA with a mean value of −1.1° and a larger change in SA in D segments, compared to C segments.
The presence of facet effusion, which is often considered an indicator of segmental instability [16], was a significant factor in predicting >10° SA correction after MIS-OLIF. No previous studies have investigated the association between the presence of facet effusion and SA correctability. Our findings suggest that facet effusion can serve as a potential indicator of satisfactory segmental correction after MIS-OLIF. The joint space occupied by fluid represents the space available within the posterior element for LL restoration, a concept that is diametrically opposed to an ankylosed facet joint [25].
Through ROC curve analysis, we established quantifiable criteria for predicting >10° SA correction after MIS-OLIF. Patients with preoperative kyphotic SA and facet effusion can expect an adequate SA correction of >10° with a 56% probability. Using our predictive formulae for the degree of SA correction, surgeons can calculate the expected degree of SA correction after MIS-OLIF. If sufficient correction is anticipated in a D segment using MIS-OLIF, surgeons can initially consider MIS-OLIF, preventing unnecessary extensive surgeries, such as posterior osteotomy or long-level fusion, based solely on preoperative sagittal imbalance in the SVA or LL. Conversely, patients with preoperative lordotic SA and no facet effusion have a 95% chance of failing to achieve an adequate SA correction >10°. Thus, for patients with lordotic SA and no preoperative facet effusion, if additional LL correction is required for remnant global sagittal imbalance, supplementary procedures, such as ACR or posterior osteotomy, may be employed during OLIF.
This study has some limitations. First, this was a retrospective study, and there is possibility of confounder bias. Second, this early-stage study did not evaluate potential mechanical complications or other long-term outcomes such as adjacent segment degeneration and cage subsidence. Moreover, we did not incorporate clinical scores such as the visual analogue scale, Oswestry disability index, or implement the Japanese Orthopaedic Association Back Pain Evaluation Questionnaire. Nevertheless, our primary aim was to identify the preoperative factors predicting >10° SA correction after MIS-OLIF. Consequently, the absence of clinical scores is unlikely to have significantly influenced our analysis. Despite these limitations, the strength of this study lies in its novelty and comprehensive examination of preoperative factors associated with satisfactory SA correction after MIS-OLIF.
Conclusion
Our study revealed that preoperative kyphotic SA and the presence of facet effusion can predict >10° SA correction following MIS-OLIF at each surgical index level. In patients with D segments showing preoperative sagittal imbalance, kyphotic SA, and facet effusion, satisfactory SA correction exceeding 10° can be expected after MIS-OLIF without ACR. Consequently, initially considering MIS-OLIF as a solitary procedure may be prudent rather than resorting to extensive interventions, such as posterior osteotomy or long-level fusion. However, for patients presenting with preoperative sagittal imbalance and lordotic SA without facet effusion, the likelihood of achieving >10° SA correction after MIS-OLIF is limited. For patients with lordotic SA and no preoperative facet effusion, supplemental procedures, such as ACR or posterior osteotomy, should be prepared for additional LL correction required for remnant global sagittal imbalance after MIS-OLIF.
References
Mittal S, Sudhakar PV, Ahuja K, et al (2023) Deformity correction with interbody fusion using lateral versus posterior approach in adult degenerative scoliosis: a systematic review and observational meta-analysis. Asian Spine J 17:431–451. https://doi.org/10.31616/asj.2022.0040/
K Hyoungmin C Bong-Soon YC Sam 2022 Pearls and pitfalls of oblique lateral interbody fusion: a comprehensive narrative review Neurospine 19 163 176 https://doi.org/10.1186/s13018-022-03084-7
Koike Y, Kotani Y, Terao H, et al (2021) Comparison of outcomes of oblique lateral interbody fusion with percutaneous posterior fixation in lateral position and minimally invasive transforaminal lumbar interbody fusion for degenerative spondylolisthesis. Asian Spine J 15:97–106. https://doi.org/10.31616/asj.2019.0342
QY Zhang J Tan K Huang 2021 Minimally invasive transforaminal lumbar interbody fusion versus oblique lateral interbody fusion for lumbar degenerative disease: a meta-analysis BMC Musculoskelet Disord 22 802 https://doi.org/10.1186/s12891-021-04687-7
Nakashima H, Kanemura T, Satake K, et al (2019) Changes in sagittal alignment following short-level lumbar interbody fusion: comparison between posterior and lateral lumbar interbody fusions. Asian Spine J 13:904–912. https://doi.org/10.31616/asj.2019.0011
J Li D Zhang Y Shen 2020 Lumbar degenerative disease after oblique lateral interbody fusion: sagittal spinopelvic alignment and its impact on low back pain J Orthop Surg Res 15 8 https://doi.org/10.1186/s13018-020-01837-w
MY Wang L Xu X Chen 2021 Optimal reconstruction of sagittal alignment according to global alignment and proportion score can reduce adjacent segment degeneration after lumbar fusion Spine 46 E257 E266 https://doi.org/10.1097/BRS.0000000000003761
Y Aoki A Nakajima H Takahashi 2015 Influence of pelvic incidence-lumbar lordosis mismatch on surgical outcomes of short-segment transforaminal lumbar interbody fusion Bmc Musculoskelet Disord 16 7 https://doi.org/10.1186/s12891-015-0676-1
G Costanzo C Zoccali P Maykowski 2014 The role of minimally invasive lateral lumbar interbody fusion in sagittal balance correction and spinal deformity Eur Spine J 23 S699 S704 https://doi.org/10.1007/s00586-014-3561-y
L Marchi L Oliveira R Amaral 2012 Anterior elongation as a minimally invasive alternative for sagittal imbalance-a case series HSS J 8 122 127 https://doi.org/10.1007/s11420-011-9226-z
Phillips FM, Isaacs RE, Rodgers WB, et al (2013) Adult degenerative scoliosis treated with XLIF: clinical and radiographical results of a prospective multicenter study with 24-month follow-up. Spine (Phila Pa 1976) 38:1853–1861. https://doi.org/10.1097/BRS.0b013e3182a43f0b
K Khajavi AY Shen 2014 Two-year radiographic and clinical outcomes of a minimally invasive, lateral, transpsoas approach for anterior lumbar interbody fusion in the treatment of adult degenerative scoliosis Eur Spine J 23 1215 1223 https://doi.org/10.1007/s00586-014-3246-6
JC Manwaring K Bach AA Ahmadian AR Deukmedjian DA Smith JS Uribe 2014 Management of sagittal balance in adult spinal deformity with minimally invasive anterolateral lumbar interbody fusion: a preliminary radiographic study J Neurosurg Spine 20 515 522 https://doi.org/10.3171/2014.2.SPINE1347
Pfirrmann CW, Metzdorf A, Zanetti M, et al (2001) Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 26:1873–1878. https://doi.org/10.1097/00007632-200109010-00011
D Weishaupt M Zanetti N Boos 1999 MR imaging and CT in osteoarthritis of the lumbar facet joints Skeletal Radiol 28 215 219 https://doi.org/10.1007/s002560050503
Chaput C, Padon D, Rush J, et al (2007) The significance of increased fluid signal on magnetic resonance imaging in lumbar facets in relationship to degenerative spondylolisthesis. Spine (Phila Pa 1976) 32:1883–1887. https://doi.org/10.1097/BRS.0b013e318113271a
D Goutallier JM Postel J Bernageau 1994 Fatty muscle degeneration in cuff ruptures - preoperative and postoperative evilation by CT scan Clin Orthop Relat Res 305 78 83 https://doi.org/10.1016/S1058-2746(99)90097-6
Schizas C, Theumann N, Burn A, et al (2010) Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine (Phila Pa 1976) 35:1919–1924. https://doi.org/10.1097/BRS.0b013e3181d359bd
S Lee JW Lee JS Yeom 2010 A Practical MRI grading system for lumbar foraminal stenosis AJR Am J Roentgenol 194 1095 1098 https://doi.org/10.2214/AJR.09.2772
Jones DHA (2010) Shenton’s line. J Bone Joint Surg Br 92-B:1312–1315. https://doi.org/10.1302/0301-620X.92B9.25094
RF Huang FM Pan WG Zhu 2022 Predictors for the restoration of the sagittal spinal malalignment in patients with lumbar stenosis after short-segment decompression and fusion surgery BMC Musculoskelet Disord 23 8 https://doi.org/10.1186/s12891-022-05666-2
Youn YH, Cho KJ, Na Y, et al (2022) Global sagittal alignment and clinical outcomes after 1–3 short-segment lumbar fusion in degenerative spinal diseases. Asian Spine J 16:551–559. https://doi.org/10.31616/asj.2021.0182
CL Zhu XB Qiu M Zhuang 2018 Surgical outcomes of single-level transforaminal lumbar interbody fusion for degenerative spondylolisthesis with and without kyphotic alignment World Neurosurg 117 E396 E402 https://doi.org/10.1016/j.wneu.2018.06.042
Alahmari A, Thornley P, Glennie A, et al (2022) Preoperative disc angle is an important predictor of segmental lordosis after degenerative spondylolisthesis fusion. Glob Spine J 10. https://doi.org/10.1177/21925682221118845
Kong MH, Morishita Y, He W, et al (2009) Lumbar segmental mobility according to the grade of the disc, the facet joint, the muscle, and the ligament pathology by using kinetic magnetic resonance imaging. Spine (Phila Pa 1976) 34:2537–2544. https://doi.org/10.1097/BRS.0b013e3181b353ea
Funding
Open Access funding enabled and organized by Seoul National University. The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, DH., Lee, J.H., Chang, BS. et al. Predicting adequate segmental lordosis correction in lumbar spinal stenosis patients undergoing oblique lumbar interbody fusion: a focus on the discontinuous segment. Eur Spine J (2024). https://doi.org/10.1007/s00586-024-08146-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00586-024-08146-4