Abstract
Purpose
To elucidate residual motion of cortical screw (CS) and pedicle screw (PS) constructs with unilateral posterior lumbar interbody fusion (ul-PLIF), bilateral PLIF (bl-PLIF), facet-sparing transforaminal lumbar interbody fusion (fs-TLIF), and facet-resecting TLIF (fr-TLIF).
Methods
A total of 35 human cadaver lumbar segments were instrumented with PS (n = 18) and CS (n = 17). Range of motion (ROM) and relative ROM changes were recorded in flexion/extension (FE), lateral bending (LB), axial rotation (AR), lateral shear (LS), anterior shear (AS), and axial compression (AC) in five instrumentational states: without interbody fusion (wo-IF), ul-PLIF, bl-PLIF, fs-TLIF, and fr-TLIF.
Results
Whereas FE, LB, AR, and AC noticeably differed between the instrumentational states, AS and LS were less prominently affected. Compared to wo-IF, ul-PLIF caused a significant increase in ROM with PS (FE + 42%, LB + 24%, AR + 34%, and AC + 77%), however, such changes were non-significant with CS. ROM was similar between wo-IF and all other interbody fusion techniques. Insertion of a second PLIF (bl-PLIF) significantly decreased ROM with CS (FE -17%, LB -26%, AR -20%, AC -51%) and PS (FE − 23%, LB − 14%, AR − 20%, AC − 45%,). Facet removal in TLIF significantly increased ROM with CS (FE + 6%, LB + 9%, AR + 17%, AC of + 23%) and PS (FE + 7%, AR + 12%, AC + 13%).
Conclusion
bl-PLIF and TLIF show similarly low residual motion in both PS and CS constructs, but ul-PLIF results in increased motion. The fs-TLIF technique is able to further decrease motion compared to fr-TLIF in both the CS and PS constructs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
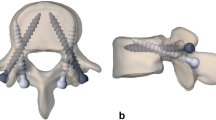
Various interbody fusion techniques are available in the spinal surgical toolbox today, with posterior lumbar interbody fusion (PLIF) and transforaminal lumbar interbody fusion (TLIF) being the most common [1, 2]. Both are usually accompanied by posterior spinal instrumentation with pedicle screws (PSs) or cortical screws (CSs) [3]. CS, also known as cortical bone trajectory, is becoming more popular because it is less invasive and offers improved screw instrumentation strength [4] (Fig. 1).
Schematic illustration of the different pedicle screw instrumentation and interbody fusion techniques. a/b Axial and lateral view of the cortical screw (CS, light gray) and pedicle screw (PS, dark gray). c Unilateral posterior lumbar interbody fusion (ul-PLIF). d Bilateral PLIF (bl-PLIF). e Facet-sparing transforaminal interbody fusion (fs-TLIF), either through the Kambin's triangle (lateral arrow) or midline approach (medial arrow). f Facet-resecting TLIF (fr-TLIF) through a left-sided facetectomy
The use of interbody fusion, especially PLIF and TLIF, has increased rapidly in the past few years [3]. The PLIF technique was first described in 1944 by Briggs and Milligan [5] and was later popularized by Cloward [6]. The use of interbody cages was later introduced by Brantigan and Steffee [7] in 1991, with superior fusion rates compared to bone grafts alone [8]. The standard PLIF techniques require insertion of two separate PLIF cages bilaterally, typically through bilateral laminotomies and, in some cases, partial resection of both facet joints to achieve adequate graft placement. During PLIF, the retraction of the thecal sac and nerve roots may endanger the nerve roots and the conus medullaris and may be technically challenging, especially in revision surgery. Thus, some authors have reported unilateral insertion of a single PLIF cage, with adequate clinical outcomes [9, 10].
To avoid these potential drawbacks of PLIF, Harms [11] described the TLIF technique, which involves the placement of a single, larger interbody cage through a unilateral facetectomy. However, unilateral resection of the facet may destabilize the instrumented segment, especially with torsional stress loadings [12]. Some authors have reported a facet-sparing TLIF approach for which increased construct stiffness is propagated [4, 13, 14].
Currently, PLIF and TLIF are considered the gold standard for circumferential fusion. However, until now, the different impact of these interbody fusion techniques on biomechanical characteristics has not been comprehensively investigated. We aimed to elucidate this gap and investigated the residual motion of CS and PS constructs of lumbar and lumbosacral segments accompanied by unilateral PLIF (ul-PLIF), bilateral PLIF (bl-PLIF), facet-sparing TLIF (fs-TLIF), and facet-resecting TLIF (fr-TLIF) (Fig. 1).
Methods
Specimen preparation
After ethical approval (BASEC Nr. 2017–00,874), we used 12 fresh-frozen human spine cadavers (Science Care, Phoenix, AZ, USA) with an average age of 59 years (range 50–68 years; 8 males and 4 females). The specimens were stored at − 20 °C until further dissection and biomechanical testing, which was performed after thawing them overnight at a room temperature of 20 °C. We removed the paraspinal muscles and connective tissues from all the specimens and divided them into L1/2, L3/4, and L5/S1 segments. Special attention was taken to not damage the intersegmental ligaments, facets, and disks.
Instrumentation
We planned all screws with optimal trajectory, screw diameter and length, half with the CS (5–6 mm diameter × 40–50 mm length) and the other half with the PS trajectory (6–7 mm diameter × 50–60 mm length) based on the computed tomography data. After 3D printing of the specimen-specific 2.7 mm drill-guides, the screws (cannulated poly-axial titanium alloy pedicle screws, Medacta International, Castel San Pietro, Switzerland) were inserted, and titanium rods were vertically attached to the screw heads. Bilateral laminotomy, removal of the supraspinous and intraspinous ligaments, and the ligamentum flavum were carried out in each segment, representing a standard midline decompression procedure. The intervertebral disk was kept intact before applying the interbody fusion techniques described below.
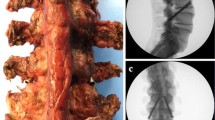
We performed the biomechanical testing of all specimens in the following constant sequence of states: (a) without interbody fusion (wo-IF); (b) nucleotomy via median anulus fibrosus and nucleus pulposus resection and unilateral PLIF cage (width x length: 12 mm × 32 mm; MectaLIF Posterior PEEK, Medacta International, Switzerland) insertion from the left; (c) insertion of a second PLIF cage from the right; (d) removal of both PLIF cages, gentle left foraminal extension of the anulus fibrosus resection if necessary and extraforaminal insertion of the TLIF cage (width x length: 14 mm × 34 mm; MectaLIF Transforaminal PEEK, Medacta International, Switzerland) from the left; and (e) resection of the left facet joint, leaving the TLIF cage in place (Fig. 2). Mean disk height and lordosis were 10 mm (range 8–14 mm) and 5° (range 1–8°) in L1/2 segments, 12 mm (range 8–15 mm) and 3° (− 2–6°) in L3/4 segments, and 11 mm (range 8–14 mm) and 5° (range 3–9°) in L5/S1 segments. We determined the accurate PLIF and TLIF cage heights (8–15 mm) based on the CT scans and “intraoperative” situation. PLIF and TLIF cage lordosis of 5° was found appropriate for all tested segments. We distracted the segments with − 100 N prior to all cage insertions and removals and compressed them with + 200 N before tightening the vertical rods.
Instrumentation following the cortical screw (CS; superior row) and pedicle screw (PS; inferior row) technique with different interbody fusion techniques: a Posterior instrumentation without interbody fusion (wo-IF). b Unilateral posterior lumbar interbody fusion (ul-PLIF). c Bilateral PLIF (bl-PLIF). d Facet-sparing transforaminal interbody fusion (fs-TLIF). e Facet-resecting TLIF (fr-TLIF) through a left sided facetectomy
Biomechanical evaluation
A previously established biomechanical setup and test protocol was used for this study [15]. The cranial and caudal vertebrae of each segment were mounted with 3D-printed clamps [16]. The test apparatus (Zwick/Roell Allroundline 10 kN, ZwickRoell GmbH & Co. KG, Germany) applied predefined loads to the cranial vertebra, while the caudal vertebra was fixed to the semi-constrained test rig (Fig. 2A). This allowed pure bending/rotational moment around the z-axis and pure shear/compression forces along the z-axis, but coupled motion in the x–y-plane was possible. The range of motion (ROM) was recorded with a motion capturing system (Fusion Track 500, Atracsys, Puidoux, Switzerland) after five preconditioning cycles were completed. Six ROM directions were tested: flexion–extension (FE), lateral bending (LB), axial rotation (AR), anterior shear (AS), lateral shear (LS), and compression-decompression (AC). In each direction, commonly used loading parameters were applied that simulate physiological conditions [15, 17]. Angular motions (FE, LB, AR) were performed with a velocity of 1°/sec, attaining a torque of ± 7.5 Nm. AS and LS were measured at 0.5 mm/sec until 150 N was attained in each direction. AC was measured at 0.1 mm/sec until + 400 N compression and – 150 N distraction were reached [17].
Statistics
We performed the statistical analysis with MATLAB (Matlab 2019a, MathWorks, Massachusetts, USA). We used non-parametric statistical testing: Mann–Whitney U test and the Wilcoxon signed rank test for unmatched and matched data, respectively. In the manuscript, we report medians along with the 25th and 75th percentiles in parentheses. Due to multiple comparisons, the Bonferroni correction of p values was applied. Statistical significance was set at α < 0.05.
Results
A total of 18 CS and 17 PS-instrumented segments were biomechanically tested and are presented here. One PS-instrumented segment had to be excluded, due to a vertebral body fracture segment. The different constructs noticeably differed in residual motion in FE, LB, AR, and AC, but AS and LS were less affected. Interbody fusion also similarly affected the CS and PS constructs, but larger differences in LB were observed in CS constructs when compared to wo-IF. Detailed information on absolute and relative ROM changes including data distribution are illustrated in Fig. 3 and Table 1 with p values in Table 2. The below reported changes in relative motion did not differ between L5/S1 and the upper lumbar segments of L1/2 and L3/4.
Change of residual range of motion (ROM) after different interbody fusion (IF) techniques in relation to posterolateral instrumentation without interbody fusion (wo-IF). Change is calculated as ΔROM (%) = (1—[ROMIF / ROMwo-IF])*100. The medians of the single differences are shown, along with the 25th and 75th percentiles. Bars below 0 indicate less motion with than without IF. Asterisks ( ∗) indicate statistical significance. CS cortical screw, PS pedicle screw, wo-IF without interbody fusion, ul-PLIF unilateral posterior lumbar interbody fusion, bl-PLIF bilateral posterior lumbar interbody fusion, fs-TLIF facet-sparing transforaminal interbody fusion, fr-TLIF facet-resecting transforaminal interbody fusion
PLIF versus TLIF
In comparison to wo-IF, ul-PLIF tended to increase the ROM, which was significant in FE with a median relative ROM change of + 42% (median absolute ROM change = + 0.4°), LB + 24% (+ 0.3°), AR + 34% (+ 0.4°), and AC + 77% (+ 0.2 mm) in PS-instrumented segments (all p < 0.05) but was not statistically significant in the CS-instrumented segments in any loading direction. In contrast, the ROM was similar and not significantly different between wo-IF and bl-PLIF in any loading direction, regardless of CS or PS instrumentation. Both fs-TLIF and fr-TLIF were also not significantly different from wo-IF in any loading direction in PS-instrumented segments. However, a significant decrease in ROM was found in LB in CS-instrumented segments both with fs-TLIF of − 29% (− 0.6 mm) and fr-TLIF of − 22% (− 0.5 mm). While ul-PLIF was inferior to TLIF in multiple loading directions in CS and PS constructs, bl-PLIF was similar and non-statistically different from both fs-TLIF and fr-TLIF.
ul-PLIF versus bl-PLIF
Insertion of a second PLIF cage reduced the ROM in all loading directions in both the CS and PS-instrumented segments, which was statistically significant, except AS in the CS-instrumented segments. In CS-instrumented segments, the largest relative ROM reductions with a second PLIF cage compared to a unilateral PLIF were found in AC of − 51% (− 0.2 mm), followed by LB − 26% (− 0.5°), AR − 20% (− 0.2°), FE − 17% (− 0.2°) LS − 11% (− 0.2 mm), and AS − 4% (− 0.1 mm). In terms of relative ROM, AC was also most affected by insertion of a second PLIF cage in PS-instrumented segments with significant relative ROM reductions of − 45% (− 0.2 mm), followed by FE − 23% (− 0.3 mm), AR − 20% (− 0.2°), and LB − 14% (− 0.4°).
fr-TLIF versus fs-TLIF
fr-TLIF was superior to fs-TLIF in both the CS and PS-instrumented segments. Removal of the facet led to a significant increase in all loading directions in CS-instrumented segments and in all loading directions except for LB and AS in PS-instrumented segments. In CS instrumentations, facet removal led to the most prominent ROM changes in relation to fs-TLIF in AC of + 23% (+ 0.1 mm), followed by AR + 17% (+ 0.3 mm), LS + 10% (+ 0.1 mm), LB 9% (+ 0.1°), FE + 6% (+ 0.1°), and AS + 6% (+ 0.1 mm). In the PS group, a relative ROM increase of fr-TLIF compared to fs-TLIF was observed in AC + 13% (+ 0.1 mm), AR + 12% (+ 0.1 mm), FE + 7% (+ 0.1°), and LS + 6% (+ 0.1 mm).
Discussion
This biomechanical study provides a comprehensive insight into residual motion with different posterior interbody fusion techniques in combination with CS and PS instrumentation. The main findings are that bilateral PLIF and TLIF provide a similar decrease in residual motion, but ul-PLIF is inferior to the traditional bl-PLIF in both PS and CS instrumentations. In contrast, the fs-TLIF technique provides more ROM reduction than the standard fr-TLIF technique and is a viable option in combination with CS and PS instrumentation.
The scientific literature on biomechanical effect of interbody fusion on segmental motion presents diverging results. For example, Harris et al. [18] reported a flexibility increase of 300% in AR following stand-alone TLIF in comparison to the intact, uninstrumented spine. Further, the study reported that a circumferential fusion with TLIF and pedicle screws did only reapproximate the flexibility to the intact, uninstrumented segment, but still an increased ROM in AR of 144% was found, with FE and LB only being decreased by 19% and 14%, respectively [18]. This stands in striking contrast to the findings of Godzik et al. [19, 20], who reported a significant motion decrease of 80% in FE and LB directions and 60% in AR when comparing PLIF and pedicle screw fixation to intact, uninstrumented segments.
When comparing interbody fusion with pedicle screw fixation to pedicle screw fixation alone, the literature also provides heterogenous findings. Whereas Godzik et al. [19] found a trend to motion decrease with the use of interbody fusion, the results of Ntilikina et al. [21] show a non-significant trend toward motion increase in FE, LB, and AR in comparison to pedicle screw instrumentation alone. Our study confirms that neither bl-PLIF nor TLIF lead to noticeable reduction of residual motion in most of the loading directions in both PS and CS instrumentations.
The posterior access route to interbody cage insertion does necessarily mean a destabilizing harm to the segment because of the focal resection of anulus fibrosus and discectomy as well as partial or full facetectomy. Further, the implanted cage may not fully replace the stabilizing properties of the intervertebral disk but also partially act as a fulcrum in FE and LB motions. This trade-off should be bared in mind in each individual case.
Anterior column support with interbody cages has been recommended to avoid pseudarthrosis and implant failure [1, 22]. In a finite-element model, interbody fusion has further shown to provide anterior column support and reduce stress on the pedicle screw-bone and screw-rod interfaces [23]. This effect has also been shown in clinical investigations, which showed a 60% reduction of early pedicle screw loosening with the use of TLIF [24]. However, regardless of the architectural structure of the spinal instrumentation construct, the mission of the interbody cage is only in part to decrease motion but to temporarily hold the disk height, until bony fusion through the impacted bone material has advanced.
PLIF versus TLIF
In our study, PLIF and TLIF were biomechanically not different, as long as PLIF was performed bilaterally. In contrast, Sim et al. [25] found higher immediate stability in lateral bending with PLIF compared to TLIF and attributed their findings to the larger interbody footprint provided by two laterally placed PLIF cages and to the destabilizing effect of the facetectomy performed with TLIF [25]. The RCT by Yang et al. [26] has shown no difference in clinical outcomes after PLIF versus TLIF for isthmic spondylolisthesis, but PLIF required more operative time and resulted in more blood loss than TLIF. The systematic review performed by Makanji et al. [27] did not find any substantial differences between PLIF and TLIF in terms of clinical outcome, with fusion rates of 94.7% and 93.3%, respectively. However, posterolateral instrumentation without interbody fusion was less successful than with interbody fusion. A recent meta-analysis comparing TLIF and PLIF found a lower complication rate, less blood loss, shorter operating time, and slightly better patient-reported outcomes with the TLIF technique in the treatment of lumbar spondylolisthesis [28].
In our opinion, the main advantage of TLIF over PLIF is the unilateral approach to the disk space, which leads to less nerve root and spinal cord retraction and therefore might also explain the lower complication rates reported. However, the choice of implant and technique is only one factor for successful fusion. In our opinion, restoring disk height and sagittal alignment, indirect neural decompression, and, most importantly, meticulous interbody bone grafting are crucial to achieve solid fusions and desirable clinical outcomes with either interbody fusion technique.
ul-PLIF versus bl-PLIF
In the face of substantial scarring, some surgeons may attempt to use only one PLIF cage intraoperatively to avoid any harm to the dura or contralateral nerve root. In a finite-element model, Zagra et al. [29] found a tendency toward higher stiffness with bl-PLIF than ul-PLIF, but ul-PLIF was comparable to TLIF in their analysis. However, the authors have shown adequate clinical results with ul-PLIF and concluded ul-PLIF as viable and safe [29]. In our study, we found substantially higher levels of residual motion with ul-PLIF compared to bl-PLIF in both CS and PS, especially in AC, FE, LB, and AR. We speculate that the focal resection of anulus fibrosus and discectomy does heavily destabilize the segment, and insertion of a single PLIF does not adequately compensate for this damage, which especially manifests in AC motion. The results of our biomechanical study support the findings of Cho et al. [30], who reported 7.7 times increased pseudarthrosis rates 1 year postoperatively with ul-PLIF compared to bl-PLIF. However, others have shown adequate clinical outcomes with the use of only one PLIF cage without radiographic signs of pseudarthrosis [10]. Based on our results, caution is advised regarding the use of ul-PLIF. In our opinion, ul-PLIF should only be considered if the operative situs does neither allow insertion of a second PLIF nor change of procedure to TLIF.
fs-TLIF versus fr-TLIF
In the first description of minimally invasive interbody fusion in 1989 by Schreiber and Leu [31], a facet-sparing approach running laterally to the facet through Kambin's triangle was described [32]. Today, fs-TLIF is increasingly reported, especially for minimally invasive interbody fusion [14, 33]. Preserving the facet in interbody fusion along with cross-link augmentation has also been recommended by Matsukawa et al. [4] as a countermeasure against increased torsional motion provided by CS constructs. The present study confirmed a reduction of residual motion with fs-TLIF compared to fr-TLIF in both CS and PS constructs, which are most prominent in AC (+ 23% and + 13%) and AR (+ 17% and + 12%) for CS and PS constructs, respectively. We believe fs-TLIF might indeed be advantageous, especially in constructs in which a large residual ROM is expected with screw instrumentation only. In our experience, an fs-TLIF with an extraforaminal approach through the Kambin's triangle can also be chosen in open surgery, especially in the upper lumbar spine, due to the more medial lying facet joints. However, in the lower lumbar spine, fs-TLIF is easily possible after midline decompression with insertion of the TLIF cage through a PLIF approach and aggressive rotation of the TLIF cage once positioned in the interbody space.
CS and interbody fusion
In this study, we had included posterior instrumentation with both PS and CS. The CS technique has gained popularity, because its trajectory beginning at the lateral part of the pars interarticularis reduces paraspinal muscle dissection and spares the superior facet joints and has further shown increased insertion torque and pull-out-strength [4, 34,35,36]. However, some authors have reported decreased construct stiffness due to the more medial running screw heads and decreased resistance to shear forces [37, 38]. Matsukawa et al. [4] have therefore recommended anterior column support with interbody fusion to overcome this potential drawback of CS in construct stability.
In this study, we have found significant reductions of residual motion with fs-TLIF and fr-TLIF in LB in the CS constructs. The reported findings support the routine use of TLIF in CS construct to decrease residual motion and increase construct stiffness. The largest clinical study to date on circumferential instrumentation with CS and TLIF by Marengo et al. [39] has also shown promising fusion rates of 94% after 1 year of follow-up.
Limitations
The biomechanical test setup in this study has some limitations. Most importantly, the reported ROM in FE, LB, AR, AS, LS, and AC is only rough simplifications of the complex motion and force patterns of a human spine in vivo. Also, the analyzed residual motions represent the immediate post-implantation motion in the postoperative short-term course, and the long-term outcome of these interbody fusion techniques was not investigated. Intuitively, the less residual motion provided with the instrumentation technique, the better the microenvironment to develop a solid bony fusion. Even though we speculate that a decrease in residual motion does have a beneficial clinical effect, we do not know to which extent the here reported differences impact the postoperative course in terms of patient satisfaction, rates of pseudarthrosis and implant loosening. In this study, we have reported both absolute and relative changes in ROM. In our opinion, the relative values are more informative because the reference ROMs, which were the instrumented segment without interbody fusion, were already low. Hence, the biomechanical effects of additional interbody fusion were also rather small with absolute differences ranging between 0–0.6° and 0–0.2 mm. However, relatively, these differences still have an essential and significant impact on residual motion.
The effect of the different interbody fusion techniques was measured on segments after bilateral laminotomy and resection of supra- and intraspinous ligaments, a common decompression technique that ensures adequate exposure of the disk space for interbody fusion. However, our results may not entirely translate to segments in which less invasive decompression techniques or minimal invasive interbody fusion were performed. With the insertion and subsequent removal of the PLIF cages from both sides, the posterior longitudinal ligament and anulus fibrosus were potentially harmed more extensively than with the direct fs-TLIF approach. This may have impaired the residual motion of the fs-TLIF construct, especially in the FE loading direction, according to a previous report that the posterior longitudinal ligament contributes 16% to flexion stability [15]. We also refrained from interbody bone grafting because we believe that this only minorly contributes to residual motion of the construct and, more importantly, is not standardizable in a biomechanical testing protocol.
Conclusion
The traditional PLIF and TLIF techniques lead to similarly low levels of residual motion in both CS and PS instrumentations. Caution is advised with the use of ul-PLIF, as it results in increased residual motion compared to bl-PLIF and TLIF and even increases motion in comparison to posterolateral instrumentation alone. In contrast, the fs-TLIF technique is able to decrease residual motion compared to the standard fr-TLIF technique in both the CS and PS constructs and presents a valuable option whenever surgically feasible.
References
Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ (2015) Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 1:2–18. https://doi.org/10.3978/j.issn.2414-469X.2015.10.05
Meng B, Bunch J, Burton D, Wang J (2021) Lumbar interbody fusion: recent advances in surgical techniques and bone healing strategies. Eur Spine J 30:22–33. https://doi.org/10.1007/s00586-020-06596-0
Reisener MJ, Pumberger M, Shue J, Girardi FP, Hughes AP (2020) Trends in lumbar spinal fusion-a literature review. J Spine Surg 6:752–761
Matsukawa K, Yato Y (2017) Lumbar pedicle screw fixation with cortical bone trajectory: a review from anatomical and biomechanical standpoints. Spine Surg Relat Res 1:164–173
Briggs H, Milligan PR (1944) Chip fusion of the low back following exploration of the spinal canal. JBJS 26:125–130
Cloward RB (1953) The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. I. indications, operative technique, after care. J Neurosurg 10:154–168. https://doi.org/10.3171/jns.1953.10.2.0154
Brantigan JW, Steffee AD, Geiger JM (1991) A carbon fiber implant to aid interbody lumbar fusion. Mech test Spine 16:S277-282. https://doi.org/10.1097/00007632-199106001-00020
Brantigan JW, Steffee AD (1993) A carbon fiber implant to aid interbody lumbar fusion. two-year clinical results in the first 26 patients. Spine 18:2106–2107. https://doi.org/10.1097/00007632-199310001-00030
Lee JH, Lee JH, Yoon KS, Kang SB, Jo CH (2008) Comparative study of unilateral and bilateral cages with respect to clinical outcomes and stability in instrumented posterior lumbar interbody fusion. Neurosurgery 63:109–113. https://doi.org/10.1227/01.Neu.0000335077.62599.F0
Fogel GR, Toohey JS, Neidre A, Brantigan JW (2007) Is one cage enough in posterior lumbar interbody fusion: a comparison of unilateral single cage interbody fusion to bilateral cages. J Spinal Disord Tech 20:60–65. https://doi.org/10.1097/01.bsd.0000211251.59953.a4
Harms JG, Jeszenszky D (1998) Die posteriore, lumbale, interkorporelle fusion in unilateraler transforaminaler technik. Oper Orthop Traumatol 10:90–102. https://doi.org/10.1007/s00064-006-0112-7
Abumi K, Panjabi MM, Kramer KM, Duranceau J, Oxland T, Crisco JJ (1990) Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine 15:1142–1147. https://doi.org/10.1097/00007632-199011010-00011
Morgenstern C, Yue JJ, Morgenstern R (2020) Full percutaneous transforaminal lumbar interbody fusion using the facet-sparing, trans-kambin approach. Clin Spine Surg 33:40–45. https://doi.org/10.1097/bsd.0000000000000827
Kim HS, Wu PH, Sairyo K, Jang IT (2021) A narrative review of uniportal endoscopic lumbar interbody fusion: comparison of uniportal facet-preserving trans-kambin endoscopic fusion and uniportal facet-sacrificing posterolateral transforaminal lumbar interbody fusion. Int J Spine Surg 15:S72-s83
Widmer J, Cornaz F, Scheibler G, Spirig JM, Snedeker JG, Farshad M (2020) Biomechanical contribution of spinal structures to stability of the lumbar spine-novel biomechanical insights. Spine J 20:1705–1716. https://doi.org/10.1016/j.spinee.2020.05.541
Cornaz F, Burkhard M, Fasser MR, Spirig JM, Snedeker JG, Farshad M, Widmer J (2021) 3D printed clamps for fixation of spinal segments in biomechanical testing. J Biomech 125:110577
Wilke HJ, Wenger K, Claes L (1998) Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur Spine J 7:148–154. https://doi.org/10.1007/s005860050045
Harris BM, Hilibrand AS, Savas PE, Pellegrino A, Vaccaro AR, Siegler S, Albert TJ (2004) Transforaminal lumbar interbody fusion: the effect of various instrumentation techniques on the flexibility of the lumbar spine. Spine (Phila Pa 1976) 29:E65–E70. https://doi.org/10.1097/01.brs.0000113034.74567.86
Godzik J, Kalb S, Reis MT, Reyes PM, Singh V, Newcomb A, Chang SW, Kelly BP, Crawford NR (2018) Biomechanical evaluation of interbody fixation with secondary augmentation: lateral lumbar interbody fusion versus posterior lumbar interbody fusion. J Spine Surg 4:180–186
Xu H, Tang H, Guan X, Jiang F, Xu N, Ju W, Zhu X, Zhang X, Zhang Q, Li M (2013) Biomechanical comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion by finite element analysis. Neurosurgery 72:21–26. https://doi.org/10.1227/NEU.0b013e3182742a69
Ntilikina Y, Charles YP, Persohn S, Skalli W (2020) Influence of double rods and interbody cages on quasistatic range of motion of the spine after lumbopelvic instrumentation. Eur spine J 29:2980–2989. https://doi.org/10.1007/s00586-020-06594-2
Mummaneni PV, Dhall SS, Eck JC, Groff MW, Ghogawala Z, Watters WC 3rd, Dailey AT, Resnick DK, Choudhri TF, Sharan A, Wang JC, Kaiser MG (2014) Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 11: interbody techniques for lumbar fusion. J Neurosurg Spine 21:67–74. https://doi.org/10.3171/2014.4.Spine14276
Xu M, Yang J, Lieberman I, Haddas R (2019) Stress distribution in vertebral bone and pedicle screw and screw-bone load transfers among various fixation methods for lumbar spine surgical alignment: a finite element study. Med Eng Phys 63:26–32. https://doi.org/10.1016/j.medengphy.2018.10.003
Kim DH, Hwang RW, Lee GH, Joshi R, Baker KC, Arnold P, Sasso R, Park D, Fischgrund J (2020) Comparing rates of early pedicle screw loosening in posterolateral lumbar fusion with and without transforaminal lumbar interbody fusion. Spine J 20:1438–1445. https://doi.org/10.1016/j.spinee.2020.04.021
Sim HB, Murovic JA, Cho BY, Lim TJ, Park J (2010) Biomechanical comparison of single-level posterior versus transforaminal lumbar interbody fusions with bilateral pedicle screw fixation: segmental stability and the effects on adjacent motion segments. J Neurosurg Spine 12:700–708. https://doi.org/10.3171/2009.12.Spine09123
Yang E-Z, Xu J-G, Liu X-K, Jin G-Y, Xiao W, Zeng B-F, Lian X-F (2016) An RCT study comparing the clinical and radiological outcomes with the use of PLIF or TLIF after instrumented reduction in adult isthmic spondylolisthesis. Eur Spine J 25:1587–1594. https://doi.org/10.1007/s00586-015-4341-z
Makanji H, Schoenfeld AJ, Bhalla A, Bono CM (2018) Critical analysis of trends in lumbar fusion for degenerative disorders revisited: influence of technique on fusion rate and clinical outcomes. Eur Spine J 27:1868–1876. https://doi.org/10.1007/s00586-018-5544-x
de Kunder SL, van Kuijk SMJ, Rijkers K, Caelers I, van Hemert WLW, de Bie RA, van Santbrink H (2017) Transforaminal lumbar interbody fusion (TLIF) versus posterior lumbar interbody fusion (PLIF) in lumbar spondylolisthesis: a systematic review and meta-analysis. Spine J 17:1712–1721. https://doi.org/10.1016/j.spinee.2017.06.018
Zagra A, Scaramuzzo L, Galbusera F, Minoia L, Archetti M, Giudici F (2015) Biomechanical and clinical study of single posterior oblique cage POLIF in the treatment of degenerative diseases of the lumbar spine. Eur Spine J 24(Suppl 7):924–930. https://doi.org/10.1007/s00586-015-4273-7
Cho JH, Hwang CJ, Lee DH, Lee CS (2021) Clinical and radiological outcomes in patients who underwent posterior lumbar interbody fusion: comparisons between unilateral and bilateral cage insertion. BMC Musculoskelet Disord 22:963. https://doi.org/10.1186/s12891-021-04852-y
Schreiber A, Leu H (1989) Restabilisation intervertebrale et arthrodese intersomatique percutanee: possibilites aujourd’hui. Rachis 1:173–179
Kambin P, Brager MD (1987) Percutaneous posterolateral discectomy: anatomy and mechanism. Clin Orthop Relat Res 223:145–154
Yamada K, Nagahama K, Abe Y, Murota E, Hiratsuka S, Takahata M, Iwasaki N (2021) Unintentional fusion in preserved facet joints without bone grafting after percutaneous endoscopic transforaminal lumbar interbody fusion. Spine Surg Relat Res 5:390–396
Matsukawa K, Kato T, Yato Y, Sasao H, Imabayashi H, Hosogane N, Asazuma T, Chiba K (2016) Incidence and risk factors of adjacent cranial facet joint violation following pedicle screw insertion using cortical bone trajectory technique. Spine 41:E851-e856. https://doi.org/10.1097/brs.0000000000001459
Matsukawa K, Yato Y, Kato T, Imabayashi H, Asazuma T, Nemoto K (2014) In vivo analysis of insertional torque during pedicle screwing using cortical bone trajectory technique. Spine 39:E240-245. https://doi.org/10.1097/brs.0000000000000116
Santoni BG, Hynes RA, McGilvray KC, Rodriguez-Canessa G, Lyons AS, Henson MA, Womack WJ, Puttlitz CM (2009) Cortical bone trajectory for lumbar pedicle screws. Spine J 9:366–373. https://doi.org/10.1016/j.spinee.2008.07.008
Perez-Orribo L, Kalb S, Reyes PM, Chang SW, Crawford NR (2013) Biomechanics of lumbar cortical screw-rod fixation versus pedicle screw-rod fixation with and without interbody support. Spine 38:635–641. https://doi.org/10.1097/BRS.0b013e318279a95e
Matsukawa K, Yato Y, Imabayashi H, Hosogane N, Asazuma T, Nemoto K (2015) Biomechanical evaluation of the fixation strength of lumbar pedicle screws using cortical bone trajectory: a finite element study. J Neurosurg Spine 23:471–478. https://doi.org/10.3171/2015.1.Spine141103
Marengo N, Berjano P, Cofano F, Ajello M, Zenga F, Pilloni G, Penner F, Petrone S, Vay L, Ducati A, Garbossa D (2018) Cortical bone trajectory screws for circumferential arthrodesis in lumbar degenerative spine: clinical and radiological outcomes of 101 cases. Eur Spine J 27:213–221. https://doi.org/10.1007/s00586-018-5599-8
Acknowledgements
The authors gratefully thank Mauro Suter for his technical support during this study. The authors further thank Medacta International (Castel San Pietro, Switzerland) for providing the implants used for this study. Imaging was performed with support of the Swiss Center for Musculoskeletal Imaging, SCMI, Balgrist Campus AG, Zurich.
Funding
Open access funding provided by University of Zurich.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors state that they have no potential conflict of interest in relation to this study's content. MF is consultant for Medacta, but unrelated to the content of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Burkhard, M.D., Spirig, J.M., Wanivenhaus, F. et al. Residual motion of different posterior instrumentation and interbody fusion constructs. Eur Spine J 32, 1411–1420 (2023). https://doi.org/10.1007/s00586-023-07597-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07597-5