Abstract
Background
Lumbar disc degeneration (LDD) may be related to aging, biomechanical and genetic factors. Despite the extensive work on understanding its etiology, there is currently no automated tool for accurate prediction of its progression.
Purpose
We aim to establish a novel deep learning-based pipeline to predict the progression of LDD-related findings using lumbar MRIs.
Materials and methods
We utilized our dataset with MRIs acquired from 1,343 individual participants (taken at the baseline and the 5-year follow-up timepoint), and progression assessments (the Schneiderman score, disc bulging, and Pfirrmann grading) that were labelled by spine specialists with over ten years clinical experience. Our new pipeline was realized by integrating the MRI-SegFlow and the Visual Geometry Group-Medium (VGG-M) for automated disc region detection and LDD progression prediction correspondingly. The LDD progression was quantified by comparing the Schneiderman score, disc bulging and Pfirrmann grading at the baseline and at follow-up. A fivefold cross-validation was conducted to assess the predictive performance of the new pipeline.
Results
Our pipeline achieved very good performances on the LDD progression prediction, with high progression prediction accuracy of the Schneiderman score (Accuracy: 90.2 ± 0.9%), disc bulging (Accuracy: 90.4% ± 1.1%), and Pfirrmann grading (Accuracy: 89.9% ± 2.1%).
Conclusion
This is the first attempt of using deep learning to predict LDD progression on a large dataset with 5-year follow-up. Requiring no human interference, our pipeline can potentially achieve similar predictive performances in new settings with minimal efforts.


Similar content being viewed by others
References
Disease GBD, Injury I, Prevalence C (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 392:1789–1858. https://doi.org/10.1016/S0140-6736(18)32279-7
Teraguchi M, Cheung JPY, Karppinen J, Bow C, Hashizume H, Luk KDK, Cheung KMC, Samartzis D (2020) Lumbar high-intensity zones on MRI: imaging biomarkers for severe, prolonged low back pain and sciatica in a population-based cohort. Spine J: Off J North Am Spine Soc 20:1025–1034. https://doi.org/10.1016/j.spinee.2020.02.015
Samartzis D, Karppinen J, Chan D, Luk KD, Cheung KM (2012) The association of lumbar intervertebral disc degeneration on magnetic resonance imaging with body mass index in overweight and obese adults: a population-based study. Arthritis Rheum 64:1488–1496. https://doi.org/10.1002/art.33462
Zehra U, Cheung JPY, Bow C, Crawford RJ, Luk KDK, Lu W, Samartzis D (2020) Spinopelvic alignment predicts disc calcification, displacement, and Modic changes: evidence of an evolutionary etiology for clinically-relevant spinal phenotypes. JOR Spine 3:e1083. https://doi.org/10.1002/jsp2.1083
Teraguchi M, Yoshimura N, Hashizume H, Yamada H, Oka H, Minamide A, Nagata K, Ishimoto Y, Kagotani R, Kawaguchi H, Tanaka S, Akune T, Nakamura K, Muraki S, Yoshida M (2017) Progression, incidence, and risk factors for intervertebral disc degeneration in a longitudinal population-based cohort: the Wakayama Spine Study. Osteoarthr Cartil 25:1122–1131. https://doi.org/10.1016/j.joca.2017.01.001
Williams FM, Popham M, Sambrook PN, Jones AF, Spector TD, MacGregor AJ (2011) Progression of lumbar disc degeneration over a decade: a heritability study. Ann Rheum Dis 70:1203–1207. https://doi.org/10.1136/ard.2010.146001
Kjaer P, Tunset A, Boyle E, Jensen TS (2016) Progression of lumbar disc herniations over an eight-year period in a group of adult Danes from the general population–a longitudinal MRI study using quantitative measures. BMC Musculoskelet Disord 17:26. https://doi.org/10.1186/s12891-016-0865-6
Zhong M, Liu JT, Jiang H, Mo W, Yu PF, Li XC, Xue RR (2017) Incidence of spontaneous resorption of lumbar disc herniation: a meta-analysis. Pain Physician 20:E45–E52
Han SS, Azad TD, Suarez PA, Ratliff JK (2019) A machine learning approach for predictive models of adverse events following spine surgery. Spine J: Off J North Am Spine Soc 19:1772–1781. https://doi.org/10.1016/j.spinee.2019.06.018
Horng MH, Kuok CP, Fu MJ, Lin CJ, Sun YN (2019) Cobb angle measurement of spine from X-Ray images using convolutional neural network. Comput Math Methods Med 2019:6357171. https://doi.org/10.1155/2019/6357171
Jin R, Luk KD, Cheung JPY, Hu Y (2019) Prognosis of cervical myelopathy based on diffusion tensor imaging with artificial intelligence methods. NMR Biomed 32:e4114. https://doi.org/10.1002/nbm.4114
Jamaludin A, Kadir T, Zisserman A (2017) SpineNet: automated classification and evidence visualization in spinal MRIs. Med Image Anal 41:63–73. https://doi.org/10.1016/j.media.2017.07.002
Jamaludin A, Kadir T, Zisserman A (2017). Self-supervised learning for spinal MRIs. In: Deep learning in medical image analysis and multimodal learning for clinical decision support, Springer, pp. 294–302.
Lootus M, Kadir T, Zisserman A (2015). Automated radiological grading of spinal MRI. In: Recent advances in computational methods and clinical applications for spine imaging, Springer, pp. 119–130.
Lu J-T, Pedemonte S, Bizzo B, Doyle S, Andriole KP, Michalski MH, Gonzalez RG, Pomerantz SR (2018). Deepspine: automated lumbar vertebral segmentation, disc-level designation, and spinal stenosis grading using deep learning. arXiv preprint:10215
Mader A, Lorenz C, Meyer C (2019). A General framework for localizing and locally segmenting correlated objects: a case study on intervertebral discs in multi-modality MR images. In: annual conference on medical image understanding and analysis, Springer, pp. 364–376.
Rouhier L, Romero FP, Cohen JP, Cohen-Adad J (2020). Spine intervertebral disc labeling using a fully convolutional redundant counting model. arXiv preprint:04387
Gros C, De Leener B, Badji A, Maranzano J, Eden D, Dupont SM, Talbott J, Zhuoquiong R, Liu Y, Granberg T (2019) Automatic segmentation of the spinal cord and intramedullary multiple sclerosis lesions with convolutional neural networks. Neuroimage 184:901–915
Han Z, Wei B, Mercado A, Leung S, Li S (2018) Spine-GAN: Semantic segmentation of multiple spinal structures. Med Image Anal 50:23–35. https://doi.org/10.1016/j.media.2018.08.005
Li X, Dou Q, Chen H, Fu C-W, Qi X, Belavý DL, Armbrecht G, Felsenberg D, Zheng G, Heng P-A (2018) 3D multi-scale FCN with random modality voxel dropout learning for intervertebral disc localization and segmentation from multi-modality MR images. Med Image Anal 45:41–54
Perone CS, Calabrese E, Cohen-Adad J (2018) Spinal cord gray matter segmentation using deep dilated convolutions. Sci Rep 8:5966. https://doi.org/10.1038/s41598-018-24304-3
Beulah A, Sharmila TS (2016). Classification of intervertebral disc on lumbar MR images using SVM. In: 2016 2nd international conference on applied and theoretical computing and communication technology (iCATccT), IEEE, pp. 293–297.
Huang S-H, Chu Y-H, Lai S-H, Novak CL (2009) Learning-based vertebra detection and iterative normalized-cut segmentation for spinal MRI. IEEE Trans Med Imaging 28:1595–1605
Chen L, Wang S, Fan W, Sun J, Naoi S (2015). Beyond human recognition: a CNN-based framework for handwritten character recognition. In: 2015 3rd IAPR Asian conference on pattern recognition (ACPR). IEEE, pp. 695–699.
LeCun Y, Bengio Y, Hinton G (2015) Deep learning 521:436–444
Cheung JP, Samartzis D, Shigematsu H, Cheung KM (2014) Defining clinically relevant values for developmental spinal stenosis: a large-scale magnetic resonance imaging study. Spine 39:1067–1076. https://doi.org/10.1097/BRS.0000000000000335
Schneiderman G, Flannigan B, Kingston S, Thomas J, Dillin WH, Watkins RG (1987) Magnetic resonance imaging in the diagnosis of disc degeneration: correlation with discography. Spine 12:276–281
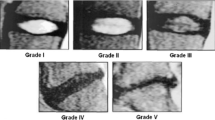
Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N (2001) Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 26:1873–1878
Xihe K, Jason P, Cheung, Honghan W, Socrates D, Teng Z (2020). MRI-SegFlow: a novel unsupervised deep learning pipeline enabling accurate vertebral segmentation of MRI images In: Proceedings of the 2020 42nd annual international conference of the IEEE engineering in medicine and biology society (EMBC). Montréal, Canada.
Chatfield K, Simonyan K, Vedaldi A, Zisserman A (2014). Return of the devil in the details: delving deep into convolutional nets; 2014. arXiv preprint: 14053531
Svensén M, Bishop CM (2007). Pattern recognition and machine learning. Springer.
Krawczyk B (2016) Learning from imbalanced data: open challenges and future directions. Prog Artif Intell 5:221–232. https://doi.org/10.1007/s13748-016-0094-0
Chawla NV, Bowyer KW, Hall LO, Kegelmeyer WP (2002) SMOTE: Synthetic minority over-sampling technique. J Artif Intell Res 16:321–357. https://doi.org/10.1613/jair.953
Liu R, Lehman J, Molino P, Such FP, Frank E, Sergeev A, Yosinski J (2018) An intriguing failing of convolutional neural networks and the coordconv solution. Advances in Neural Information Processing Systems, p. 9605–9616. arXiv preprint 1807.03247
Day O, Khoshgoftaar TM (2017) A survey on heterogeneous transfer learning. J Big Data 4(1):1–42
Acknowledgements
We would like to thank the Hong Kong Theme-Based Research Scheme (T12-708/12N) for supporting the establishment of the MRI dataset. We would like to thank the Innovation and Technology Commission Seed Fund (ITS/404/18) for supporting the equipment used in this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors decare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: implantation process
Appendix: implantation process
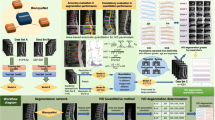
Network architecture
The basic architecture of VGG-M [30] is adopted in our pipeline, which can be divided into two parts, the encoder and the classifier (Fig. 2). The encoder consists of a stack of alternately arranged convolutional layers and maxpooling layers [25], which is trained to extract the hierarchical feature from the input disc region. The classifier consists of two fully connected layers, which is trained to produce the pathology grade prediction based on the feature extracted by the encoder. The Rectified Linear Unit (ReLU) is served as the activation function of all convolutional and fully connected layers of the network, except the output, which is activated by the softmax function.
The input of our deep learning network is the disc region of a lumbar MRI, which is resized to \(150\times 200\times 9\), where \(9\) represents the middle \(9\) slices of the MRI series. The output of the network is the probability prediction of each follow-up disc pathology grade, which is an \(1\times 5\) array for the Pfirrmann grading and an \(1\times 4\) array for the disc bulge or the Schneiderman score. The final grade prediction is the grade with the highest probability.
Since the original VGG-M is designed for the classification of natural images and has a large-scale dataset for the training process, two modifications are introduced in the network architecture for our specific task. First, the coordinate channels [34] are introduced in the input, which provide the relative location information and improve the position sensitivity of our network. Besides, the number of network parameters is reduced to accelerate the training process. More specifically, the channel numbers of the convolutional layers with the scale of \(5\times 5\) and \(3\times 3\) are reduced from 256 and 512 to 128 and 256, respectively, therefore the total number of network parameter is reduced from 6.5 to 2.8 M [30].
Training strategy
The basic idea of transfer learning [35] is adopted in the training process of the model, which is divided into two steps, pretraining and finetuning. In the pretraining step, the network is trained from the scratch for the pathology classification, which aims to enable the encoder of the network to extract the key features for the specific pathology from MRI. The label used in the pretraining step is the baseline disc pathology grade. Then, in the finetuning process, the network is further trained for pathology prediction, and the follow-up grade label is used to provide supervision. The parameters of the encoder in the pretrained network are preserved to inherit the ability of feature extraction, while the classifier is reinitialized for the new prediction task. The random on-the-fly data augmentation strategy is adopted in both training steps to reduce the risk of overfitting, which includes: (i) translation of \(\pm 15\mathrm{\%}\times \mathrm{w}\) in x-axis and \(\pm 10\mathrm{\%}\times \mathrm{w}\) in y-axis, where \(\mathrm{w}\) represents the average width of the disc region (ii) rotation with \(\pm 5^\circ \) (iii) rescaling with \(1\pm 10\mathrm{\%}\) scaling factor.
Implementation details
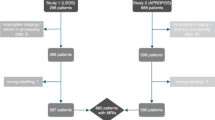
13,130 disc samples are extracted from 2686 MRIs by the MRI-SegFlow for the validation of our method, and 6565 of them have the label of the follow-up pathology grades. The fivefold cross-validation strategy is employed. The samples with the follow-up grade labelled are equally divided into 5 subgroups. In each round of validation, one subgroup is selected as testing data and the other 4 subgroups combining with the samples without labels of follow-up grade are served as the training data. All training data are used in the pretraining step, while only the samples with the follow-up labels are used in the finetuning step. The testing data are invisible for the CNN model in the whole training process.
The mini-batch strategy is adopted in both two training steps with the batch size of 256 for pretraining and 64 for finetuning. The model reaches convergence in about 300 training epochs in the pretraining step and 100 in the finetuning step. In both steps, the learning rate is 0.001, Mean squared error is served as loss and the optimizer is Stochastic gradient descent. The data augmentation is applied in both training steps. TensorFlow 2.0 was used to implement the model with NVIDIA 2080Ti.
Rights and permissions
About this article
Cite this article
Cheung, J.P.Y., Kuang, X., Lai, M.K.L. et al. Learning-based fully automated prediction of lumbar disc degeneration progression with specified clinical parameters and preliminary validation. Eur Spine J 31, 1960–1968 (2022). https://doi.org/10.1007/s00586-021-07020-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-021-07020-x