Abstract
Purpose
The loss of nutrient supply is a suspected contributor of intervertebral disc degeneration. However, the extent to which low nutrition affects disc annulus fibrosus (AF) cells is unknown as nutrient deprivation has mainly been investigated in disc nucleus pulposus cells. Hence, an experimental study was designed to clarify the effects of limited nutrients on disc AF cell fate, including autophagy, the process by which cells recycle their own damaged components.
Methods
Rabbit disc AF cells were cultured in different media with varying serum concentrations under 5% oxygen. Cellular responses to changes in serum and nutrient concentrations were determined by measuring proliferation and metabolic activity. Autophagic flux in AF cells was longitudinally monitored using imaging cytometry and Western blotting for LC3, HMGB1, and p62/SQSTM1. Apoptosis (TUNEL staining and cleaved caspase-3 immunodetection) and cellular senescence (senescence-associated β-galactosidase assay and p16/INK4A immunodetection) were measured.
Results
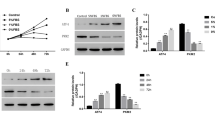
Markers of apoptosis and senescence increased, while cell proliferation and metabolic activity decreased under the withdrawal of serum and of nutrients other than oxygen, confirming cellular stress. Time-dependent increases in autophagy markers, including LC3 puncta number per cell, LC3-II expression, and cytoplasmic HMGB1, were observed under conditions of reduced nutrition, while an autophagy substrate, p62/SQSTM1, decreased over time. Collectively, these findings suggest increased autophagic flux in disc AF cells under serum and nutrient deprivation.
Conclusion
Disc AF cells exhibit distinct responses to serum and nutrient deprivation. Cellular responses include cell death and quiescence in addition to reduced proliferation and metabolic activity, as well as activation of autophagy under conditions of nutritional stress.
Graphical abstract
These slides can be retrieved under Electronic Supplementary Material.







Similar content being viewed by others
References
Urban JP, Smith S, Fairbank JC (2004) Nutrition of the intervertebral disc. Spine (Phila Pa 1976) 29(23):2700–2709
Fuchs Y, Steller H (2011) Programmed cell death in animal development and disease. Cell 147(4):742–758. https://doi.org/10.1016/j.cell.2011.10.033
Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW (2005) DNA repair, genome stability, and aging. Cell 120(4):497–512. https://doi.org/10.1016/j.cell.2005.01.028
Le Maitre CL, Freemont AJ, Hoyland JA (2007) Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther 9(3):R45. https://doi.org/10.1186/ar2198
Yurube T, Hirata H, Kakutani K, Maeno K, Takada T, Zhang Z, Takayama K, Matsushita T, Kuroda R, Kurosaka M, Nishida K (2014) Notochordal cell disappearance and modes of apoptotic cell death in a rat tail static compression-induced disc degeneration model. Arthritis Res Ther 16(1):R31. https://doi.org/10.1186/ar4460
Vo NV, Hartman RA, Patil PR, Risbud MV, Kletsas D, Iatridis JC, Hoyland JA, Le Maitre CL, Sowa GA, Kang JD (2016) Molecular mechanisms of biological aging in intervertebral discs. J Orthop Res 34(8):1289–1306. https://doi.org/10.1002/jor.23195
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132(1):27–42. https://doi.org/10.1016/j.cell.2007.12.018
Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, Adhihetty PJ, Adler SG, Agam G, Agarwal R, Aghi MK, Agnello M, Agostinis P, Aguilar PV, Aguirre-Ghiso J, Airoldi EM, Ait-Si-Ali S, Akematsu T, Akporiaye ET, Al-Rubeai M, Albaiceta GM et al (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12(1):1–222. https://doi.org/10.1080/15548627.2015.1100356
Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ 3rd, Lotze MT (2010) Endogenous HMGB1 regulates autophagy. J Cell Biol 190(5):881–892. https://doi.org/10.1083/jcb.200911078
Ni BB, Li B, Yang YH, Chen JW, Chen K, Jiang SD, Jiang LS (2014) The effect of transforming growth factor beta1 on the crosstalk between autophagy and apoptosis in the annulus fibrosus cells under serum deprivation. Cytokine 70(2):87–96. https://doi.org/10.1016/j.cyto.2014.07.249
Miyazaki S, Kakutani K, Yurube T, Maeno K, Takada T, Zhang Z, Kurakawa T, Terashima Y, Ito M, Ueha T, Matsushita T, Kuroda R, Kurosaka M, Nishida K (2015) Recombinant human SIRT1 protects against nutrient deprivation-induced mitochondrial apoptosis through autophagy induction in human intervertebral disc nucleus pulposus cells. Arthritis Res Ther 17:253. https://doi.org/10.1186/s13075-015-0763-6
Ito M, Yurube T, Kakutani K, Maeno K, Takada T, Terashima Y, Kakiuchi Y, Takeoka Y, Miyazaki S, Kuroda R, Nishida K (2017) Selective interference of mTORC1/RAPTOR protects against human disc cellular apoptosis, senescence, and extracellular matrix catabolism with Akt and autophagy induction. Osteoarthritis Cartilage 25(12):2134–2146. https://doi.org/10.1016/j.joca.2017.08.019
Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N (2001) Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 26(17):1873–1878
Kofoed H, Sjontoft E, Siemssen SO, Olesen HP (1985) Bone marrow circulation after osteotomy. Blood flow, pO2, pCO2, and pressure studied in dogs. Acta Orthop Scand 56(5):400–403
Kamada S, Kikkawa U, Tsujimoto Y, Hunter T (2005) Nuclear translocation of caspase-3 is dependent on its proteolytic activation and recognition of a substrate-like protein(s). J Biol Chem 280(2):857–860. https://doi.org/10.1074/jbc.C400538200
Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE (2004) Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114(9):1299–1307. https://doi.org/10.1172/JCI22475
Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119(3):493–501
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O et al (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 92(20):9363–9367
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418(6894):191–195. https://doi.org/10.1038/nature00858nature00858
Risbud MV, Fertala J, Vresilovic EJ, Albert TJ, Shapiro IM (2005) Nucleus pulposus cells upregulate PI3K/Akt and MEK/ERK signaling pathways under hypoxic conditions and resist apoptosis induced by serum withdrawal. Spine (Phila Pa 1976) 30(8):882–889
Johnson WE, Stephan S, Roberts S (2008) The influence of serum, glucose and oxygen on intervertebral disc cell growth in vitro: implications for degenerative disc disease. Arthritis Res Ther 10(2):R46. https://doi.org/10.1186/ar2405
Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D et al (2015) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14(4):644–658. https://doi.org/10.1111/acel.12344
Robles SJ, Adami GR (1998) Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene 16(9):1113–1123. https://doi.org/10.1038/sj.onc.1201862
Yang NC, Hu ML (2005) The limitations and validities of senescence associated-beta-galactosidase activity as an aging marker for human foreskin fibroblast Hs68 cells. Exp Gerontol 40(10):813–819. https://doi.org/10.1016/j.exger.2005.07.011
Acknowledgements
The authors thank Drs. Thomas P. Lozito (Department of Orthopaedic Surgery, Center for Cellular and Molecular Engineering, University of Pittsburgh, Pittsburgh, PA), Mayumi Morizane (Department of Obstetrics, Gynecology and Reproductive Sciences, Magee-Womens Research Institute, University of Pittsburgh, Pittsburgh, PA), Masahiro Shuda (Cancer Virology Program, University of Pittsburgh Cancer Institute, Pittsburgh, PA), and Tetsuya Watanabe (Division of Pulmonary, Allergy and Critical Care Medicine, Department of Medicine, University of Pittsburgh Asthma Institute, University of Pittsburgh, Pittsburgh, PA) for their expertise. We also thank Mr. Kevin Ngo and Ms. Qing Dong (Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, PA) for their technical assistance. This work was supported in part by The Albert B. Ferguson, Jr., M.D. Orthopaedic Fund of The Pittsburgh Foundation, NIH AG044376, and The Uehara Memorial Foundation (Grant Nos. AG044376, 2012400067).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
TY, WB, HM, RH, KT, YK, KN, MK, NV, ML, and GS have no conflicts of interest to declare. JDK has received research grants from Stryker and Synthes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yurube, T., Buchser, W.J., Moon, H.J. et al. Serum and nutrient deprivation increase autophagic flux in intervertebral disc annulus fibrosus cells: an in vitro experimental study. Eur Spine J 28, 993–1004 (2019). https://doi.org/10.1007/s00586-019-05910-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-019-05910-9