Abstract
Study design and objective
The purpose of this prospective clinical study is to evaluate the clinical and radiographic outcomes using a silicate-substituted calcium phosphate (Si-CaP) as a bone graft substitute in surgery for adolescent idiopathic scoliosis (AIS).
Summary of background data
In posterior corrective surgery for AIS, harvesting autologous bone from the iliac crest still represents the gold standard to augment the local bone graft though it is comparatively invasive and associated with donor site morbidity. Si-CaP enriched with bone marrow aspirate (BMA) might be an appropriate bone graft extender to overcome these difficulties.
Methods
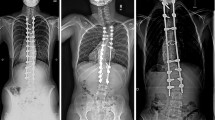
Eighteen female and three male patients with AIS who underwent corrective posterior instrumentation were observed clinically and radiographically for a minimum of 24 months. In all cases, 20–40 ml Si-CaP granules (ACTIFUSE) mixed with BMA from vertebral bodies was used to extend the local bone graft. Fusion was assessed by standardized conventional radiographs regarding loss of correction and implant failure. Clinical outcome was evaluated with use of the Scoliosis Research Society-22 patient Questionnaire (SRS-22) and a Visual Analog Scale (VAS) for back pain.
Results
Cobb angle of major curves averaged 63° preoperatively, 22° after surgery, and 24° at final follow-up, with a maximum loss of correction of 7° recorded after 4 months. No adverse effects related to the study material had been observed. In all patients, there was no evidence of implant failure, and formation of an increasingly densifying ‘fusion mass’ was visible, as assessed by conventional radiography. VAS score for back pain averaged 1.7 before surgery, 2.3 at discharge, and 1.5 at final follow-up. Outcome assessment using the SRS-22 revealed a significantly enhanced overall health-related quality of life (84 vs. 74 % before surgery; P = 0.0005) due to a significant improvement of the domains ‘self image’ (77 vs. 59 %; P = 0.0002) and ‘pain’ (88 vs. 80 %; P = 0.02). Patients’ management satisfaction averaged 93 %.
Conclusions
Si-CaP augmented with BMA from vertebral bodies seems to prove an effective, safe, and easy to handle bone graft extender in scoliosis surgery and thus a suitable alternative to bone harvesting procedures.





Similar content being viewed by others
References
Sengupta DK, Truumees E, Patel CK, Kazmierczak C, Hughes B, Elders G, Herkowitz HN (2006) Outcome of local bone versus autogenous iliac crest bone graft in the instrumented posterolateral fusion of the lumbar spine. Spine 31:985–991
Kessler P, Thorwarth M, Bloch-Birkholz A, Nkenke E, Neukam FW (2005) Harvesting of bone from the iliac crest—comparison of the anterior and posterior sites. Br J Oral Maxillofac Surg 43:51–56
Fernyhough JC, Schimandle JJ, Weigel MC, Edwards CC, Levine AM (1992) Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine 17:1474–1480
Banwart JC, Asher MA, Hassanein RS (1995) Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine 20:1055–1060
Skaggs DL, Samuelson MA, Hale JM, Kay RM, Tolo VT (2000) Complications of posterior iliac crest bone grafting in spine surgery in children. Spine 25:2400–2402
Robertson PA, Wray AC (2001) Natural history of posterior iliac crest bone graft donation for spinal surgery: a prospective analysis of morbidity. Spine 26:1473–1476
Bridwell KH, O’Brien MF, Lenke LG, Baldus C, Blanke K (1994) Posterior spinal fusion supplemented with only allograft bone in paralytic scoliosis. Does it work? Spine (Phila Pa 1976) 19:2658–2666
Yazici M, Asher MA (1997) Freeze-dried allograft for posterior spinal fusion in patients with neuromuscular spinal deformities. Spine (Phila Pa 1976) 22:1467–1471
Jones KC, Andrish J, Kuivila T, Gurd A (2002) Radiographic outcomes using freeze-dried cancellous allograft bone for posterior spinal fusion in pediatric idiopathic scoliosis. J Pediatr Orthop 22:285–289
Knapp DR Jr, Jones ET, Blanco JS, Flynn JC, Price CT (2005) Allograft bone in spinal fusion for adolescent idiopathic scoliosis. J Spinal Disord Tech 18(Suppl):S73–S76
An HS, Lynch K, Toth J (1995) Prospective comparison of autograft vs. allograft for adult posterolateral lumbar spine fusion: differences among freeze-dried, frozen, and mixed grafts. J Spinal Disord 8:131–135
Jorgenson SS, Lowe TG, France J, Sabin J (1994) A prospective analysis of autograft versus allograft in posterolateral lumbar fusion in the same patient. A minimum of 1-year follow-up in 144 patients. Spine 19:2048–2053
Ehrler DM, Vaccaro AR (2000) The use of allograft bone in lumbar spine surgery. Clin Orthop Relat Res 371:38–45
Price CT, Connolly JF, Carantzas AC, Ilyas I (2003) Comparison of bone grafts for posterior spinal fusion in adolescent idiopathic scoliosis. Spine 28:793–798
Buck BE, Resnick L, Shah SM (1990) Human immunodeficiency virus cultured from bone. Implications for transplantation. Clin Orthop Relat Res 251:249–253
Journeaux SF, Johnson N, Bryce SL, Friedman SJ, Sommerville SM, Morgan DA (1999) Bacterial contamination rates during bone allograft retrieval. J Arthroplasty 14:677–681
Woolf SK, Gross RH (2001) Perceptions of allograft safety and efficacy among spinal deformity surgeons. J Pediatr Orthop 21:767–771
Barriga A, Diaz-de-Rada P, Barroso JL, Alfonso M, Lamata M, Hernaez S, Beguiristain JL, San-Julian M, Villas C (2004) Frozen cancellous bone allografts: positive cultures of implanted grafts in posterior fusions of the spine. Eur Spine J 13:152–156
Crawford MJ, Swenson CL, Arnoczky SP, O’Shea J, Ross H (2004) Lyophilization does not inactivate infectious retrovirus in systemically infected bone and tendon allografts. Am J Sports Med 32:580–586
Eastlund T (2006) Bacterial infection transmitted by human tissue allograft transplantation. Cell Tissue Bank 7:147–166
Bucholz RW (2002) Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res 395:44–52
Erbe EM, Marx JG, Clineff TD, Bellincampi LD (2001) Potential of an ultraporous beta-tricalcium phosphate synthetic cancellous bone void filler and bone marrow aspirate composite graft. Eur Spine J 10(Suppl 2):S141–S146
Betz RR (2002) Limitations of autograft and allograft: new synthetic solutions. Orthopedics 25:s561–s570
McLain RF, Fleming JE, Boehm CA, Muschler GF (2005) Aspiration of osteoprogenitor cells for augmenting spinal fusion: comparison of progenitor cell concentrations from the vertebral body and iliac crest. J Bone Joint Surg Am 87:2655–2661
Muschler GF, Matsukura Y, Nitto H, Boehm CA, Valdevit AD, Kambic HE, Davros WJ, Easley KA, Powell KA (2005) Selective retention of bone marrow-derived cells to enhance spinal fusion. Clin Orthop Relat Res 432:242–251
McLain RF, Boehm CA, Rufo-Smith C, Muschler GF (2009) Transpedicular aspiration of osteoprogenitor cells from the vertebral body: progenitor cell concentrations affected by serial aspiration. Spine J 9:995–1002
Le Huec JC, Lesprit E, Delavigne C, Clement D, Chauveaux D, Le Rebeller A (1997) Tri-calcium phosphate ceramics and allografts as bone substitutes for spinal fusion in idiopathic scoliosis as bone substitutes for spinal fusion in idiopathic scoliosis: comparative clinical results at four years. Acta Orthop Belg 63:202–211
Ransford AO, Morley T, Edgar MA, Webb P, Passuti N, Chopin D, Morin C, Michel F, Garin C, Pries D (1998) Synthetic porous ceramic compared with autograft in scoliosis surgery. A prospective, randomized study of 341 patients. J Bone Joint Surg Br 80:13–18
Cavagna R, Daculsi G, Bouler JM (1999) Macroporous calcium phosphate ceramic: a prospective study of 106 cases in lumbar spinal fusion. J Long Term Eff Med Implants 9:403–412
Delecrin J, Takahashi S, Gouin F, Passuti N (2000) A synthetic porous ceramic as a bone graft substitute in the surgical management of scoliosis: a prospective, randomized study. Spine 25:563–569
Muschik M, Ludwig R, Halbhubner S, Bursche K, Stoll T (2001) Beta-tricalcium phosphate as a bone substitute for dorsal spinal fusion in adolescent idiopathic scoliosis: preliminary results of a prospective clinical study. Eur Spine J 10(Suppl 2):S178–S184
Meadows GR (2002) Adjunctive use of ultraporous beta-tricalcium phosphate bone void filler in spinal arthrodesis. Orthopedics 25:s579–s584
Epstein NE (2006) A preliminary study of the efficacy of beta tricalcium phosphate as a bone expander for instrumented posterolateral lumbar fusions. J Spinal Disord Tech 19:424–429
Moro-Barrero L, Acebal-Cortina G, Suarez–Suarez M, Perez-Redondo J, Murcia-Mazon A, Lopez-Muniz A (2007) Radiographic analysis of fusion mass using fresh autologous bone marrow with ceramic composites as an alternative to autologous bone graft. J Spinal Disord Tech 20:409–415
Lerner T, Bullmann V, Schulte TL, Schneider M, Liljenqvist U (2009) A level-1 pilot study to evaluate of ultraporous beta-tricalcium phosphate as a graft extender in the posterior correction of adolescent idiopathic scoliosis. Eur Spine J 18:170–179
Hing KA, Annaz B, Saeed S, Revell PA, Buckland T (2005) Microporosity enhances bioactivity of synthetic bone graft substitutes. J Mater Sci Mater Med 16:467–475
Patel N, Best SM, Bonfield W, Gibson IR, Hing KA, Damien E, Revell PA (2002) A comparative study on the in vivo behavior of hydroxyapatite and silicon substituted hydroxyapatite granules. J Mater Sci Mater Med 13:1199–1206
Hing KA, Revell PA, Smith N, Buckland T (2006) Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials 27:5014–5026
Porter AE, Buckland T, Hing K, Best SM, Bonfield W (2006) The structure of the bond between bone and porous silicon-substituted hydroxyapatite bioceramic implants. J Biomed Mater Res A 78:25–33
Hing KA, Wilson LF, Buckland T (2007) Comparative performance of three ceramic bone graft substitutes. Spine J 7:475–490
Wheeler DL, Jenis LG, Kovach ME, Marini J, Turner AS (2007) Efficacy of silicated calcium phosphate graft in posterolateral lumbar fusion in sheep. Spine J 7:308–317
Lenke LG, Betz RR, Harms J, Bridwell KH, Clements DH, Lowe TG, Blanke K (2001) Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am 83-A:1169–1181
Cobb J (1948) Outline for the study of scoliosis. In: Instructional course letters, vol 5. American Academy of Orthopaedic Surgeons, Ann Arbor
Betz RR, Petrizzo AM, Kerner PJ, Falatyn SP, Clements DH, Huss GK (2006) Allograft versus no graft with a posterior multisegmented hook system for the treatment of idiopathic scoliosis. Spine 31:121–127
Haher TR, Gorup JM, Shin TM, Homel P, Merola AA, Grogan DP, Pugh L, Lowe TG, Murray M (1999) Results of the Scoliosis Research Society instrument for evaluation of surgical outcome in adolescent idiopathic scoliosis. A multicenter study of 244 patients. Spine (Phila Pa 1976) 24:1435–1440
Asher M, Min Lai S, Burton D, Manna B (2003) The reliability and concurrent validity of the scoliosis research society-22 patient questionnaire for idiopathic scoliosis. Spine (Phila Pa 1976) 28:63–69
Niemeyer T, Schubert C, Halm HF, Herberts T, Leichtle C, Gesicki M (2009) Validity and reliability of an adapted german version of scoliosis research society-22 questionnaire. Spine (Phila Pa 1976) 34:818–821
Lerner T, Griefingholt H, Liljenqvist U (2009) Bone substitutes in scoliosis surgery. Orthopade 38:181–188
Korovessis P, Koureas G, Zacharatos S, Papazisis Z, Lambiris E (2005) Correlative radiological, self-assessment and clinical analysis of evolution in instrumented dorsal and lateral fusion for degenerative lumbar spine disease. Autograft versus coralline hydroxyapatite. Eur Spine J 14:630–638
Violas P, Chapuis M, Bracq H (2004) Local autograft bone in the surgical management of adolescent idiopathic scoliosis. Spine 29:189–192
Betz RR, Lavelle WF, Samdani AF (2010) Bone grafting options in children. Spine (Phila Pa 1976) 35:1648–1654
Maeda T, Buchowski JM, Kim YJ, Mishiro T, Bridwell KH (2009) Long adult spinal deformity fusion to the sacrum using rhBMP-2 versus autogenous iliac crest bone graft. Spine (Phila Pa 1976) 34:2205–2212
Mulconrey DS, Bridwell KH, Flynn J, Cronen GA, Rose PS (2008) Bone morphogenetic protein (RhBMP-2) as a substitute for iliac crest bone graft in multilevel adult spinal deformity surgery: minimum two-year evaluation of fusion. Spine (Phila Pa 1976) 33:2153–2159
Conflict of interest
This study was supported financially by ApaTech LTD (Elstree, Herfordshire, UK).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lerner, T., Liljenqvist, U. Silicate-substituted calcium phosphate as a bone graft substitute in surgery for adolescent idiopathic scoliosis. Eur Spine J 22 (Suppl 2), 185–194 (2013). https://doi.org/10.1007/s00586-012-2485-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-012-2485-7