Abstract
Purpose
Intervertebral disc degeneration is considered to be a major feature of low back pain. Furthermore, oxidative stress has been shown to be an important factor in degenerative diseases such as osteoarthritis and is considered a cause of intervertebral disc degeneration. The purpose of this study was to clarify the correlation between oxidative stress and intervertebral disc degeneration using Broad complex-Tramtrack-Bric-a-brac and cap‘n’collar homology 1 deficient (Bach 1−/−) mice which highly express heme oxygenase-1 (HO-1). HO-1 protects cells from oxidative stress.
Methods
Caudal discs of 12-week-old and 1-year-old mice were evaluated as age-related models. Each group and period, 5 mice (a total of 20 mice, a total of 20 discs) were evaluated as age-related model. C9–C10 caudal discs in 12-week-old Bach 1−/− and wild-type mice were punctured using a 29-gauge needle as annulus puncture model. Each group and period, 5 mice (a total of 60 mice, a total of 60 discs) were evaluated. The progress of disc degeneration was evaluated at pre-puncture, 1, 2, 4, 8 and 12 weeks post-puncture. Radiographic, histologic and immunohistologic analysis were performed to compare between Bach 1−/− and wild-type mice.
Results
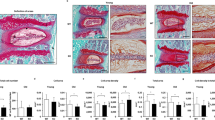
In the age-related model, there were no significant differences between Bach 1−/− and wild-type mice radiologically and histologically. However, in the annulus puncture model, histological scoring revealed significant difference at 8 and 12 weeks post-puncture. The number of HO-1 positive cells was significantly greater in Bach 1−/− mice at every period. The apoptosis rate was significantly lower at 1 and 2 weeks post-puncture in Bach 1−/− mice.
Conclusions
Oxidative stress prevention may avoid the degenerative process of the intervertebral disc after puncture, reducing the number of apoptosis cells. High HO-1 expression may also inhibit oxidative stress and delay the process of intervertebral disc degeneration.










Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Kirkaldy-Willis WH, Farfan HF (1982) Instability of the lumbar spine. Clin Orthop Relat Res 165:110–123
Urban JP, Roberts S (2003) Degeneration of the intervertebral disc. Arthritis Res Ther 5:120–130
Tanaka N, An HS, Lim TH et al (2001) The relationship between disc degeneration and flexibility of the lumbar spine. Spine J 1:47–56
An HS, Masuda K, Inoue N (2006) Intervertebral disc degeneration: biological and biomechanical factors. J Orthop Sci 11:541–552
Seki S, Kawaguchi Y, Chiba K et al (2005) A functional SNP in CILP, encoding cartilage intermediate layer protein, is associated with susceptibility to lumbar disc disease. Nat Genet 37:607–612
Kim KW, Ha KY, Lee JS et al (2007) The apoptotic effects of oxidative stress and antiapoptotic effects of caspase inhibitors on rat notochordal cells. Spine 32:2443–2448
Poveda L, Hottiger M, Boos N et al (2009) Peroxynitrite induces gene expression in intervertebral disc cells. Spine 34:1127–1133
Kikuchi G, Yoshida T, Noguchi M (2005) Heme oxygenase and heme degradation. Biochem Biophys Res Commun 338:558–567
Shan Y, Lambrecht RW, Ghaziani T et al (2004) Role of Bach-1 in regulation of heme oxygenase-1 in human liver cells: insights from studies with small interfering RNAS. J Biol Chem 279:51769–51774
Shibahara S, Muller RM, Taguchi H (1987) Transcriptional control of rat heme oxygenase by heat shock. J Biol Chem 262:12889–12892
Alam J, Shibahara S, Smith A (1989) Transcriptional activation of the heme oxygenase gene by heme and cadmium in mouse hepatoma cells. J Biol Chem 264:6371–6375
Sun J, Hoshino H, Takaku K et al (2002) Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J 21:5216–5224
Poss KD, Tonegawa S (1997) Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA 94:10925–10930
Hancock WW, Buelow R, Sayegh MH et al (1998) Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nat Med 4:1392–1396
Odaka Y, Takahashi T, Yamasaki A et al (2000) Prevention of halothane-induced hepatotoxicity by hemin pretreatment: protective role of heme oxygenase-1 induction. Biochem Pharmacol 59:871–880
Sun J, Brand M, Zenke Y, Tashiro S, Groudine M, Igarashi K (2004) Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network. Proc Natl Acad Sci U S A 101:1461–1466
Ochiai S, Mizuno T, Deie M et al (2008) Oxidative stress reaction in the meniscus of Bach 1 deficient mice: potential prevention of meniscal degeneration. J Orthop Res 26:894–898
Lu DS, Shono Y, Oda I et al (1997) Effects of chondroitinase ABC and chymopapain on spinal motion segment biomechanics. An in vivo biomechanical, radiologic, and histologic canine study. Spine 22:1828–1834 (discussion 34–35)
Boos N, Weissbach S, Rohrbach H et al (2002) Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine 27:2631–2644
Yang F, Leung VY, Luk KD et al (2009) Injury-induced sequential transformation of notochordal nucleus pulposus to chondrogenic and fibrocartilaginous phenotype in the mouse. J Pathol 218:113–121
Han B, Zhu K, Li FC et al (2008) A simple disc degeneration model induced by percutaneous needle puncture in the rat tail. Spine 33:1925–1934
Masuda K, Aota Y, Muehleman C et al (2005) A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine 30:5–14
Yamada K, Tanaka N, Nakanishi K et al (2008) Modulation of the secondary injury process after spinal cord injury in Bach1-deficient mice by heme oxygenase-1. J Neurosurg Spine 9:611–620
Davies CM, Guilak F, Weinberg JB et al (2008) Reactive nitrogen and oxygen species in interleukin-1-mediated DNA damage associated with osteoarthritis. Osteoarthritis Cartilage 16:624–630
Lotz JC (2004) Animal models of intervertebral disc degeneration: lessons learned. Spine 29:2742–2750
Olmarker K (2008) Puncture of a lumbar intervertebral disc induces changes in spontaneous pain behavior: an experimental study in rats. Spine 33:850–855
Elliott DM, Sarver JJ (2004) Young investigator award winner: validation of the mouse and rat disc as mechanical models of the human lumbar disc. Spine 29:713–722
Sarver JJ, Elliott DM (2005) Mechanical differences between lumbar and tail discs in the mouse. J Orthop Res 23:150–155
Ariga K, Miyamoto S, Nakase T et al (2001) The relationship between apoptosis of endplate chondrocytes and aging and degeneration of the intervertebral disc. Spine 26:2414–2420
Acknowledgments
This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology-Japan (No. 20591743). The manuscript submitted does not contain information about medical device(s)/drug(s). Foundation funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohta, R., Tanaka, N., Nakanishi, K. et al. Heme oxygenase-1 modulates degeneration of the intervertebral disc after puncture in Bach 1 deficient mice. Eur Spine J 21, 1748–1757 (2012). https://doi.org/10.1007/s00586-012-2442-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-012-2442-5