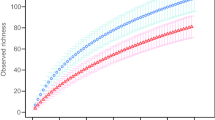
Abstract
Neotropical montane forests are often dominated by ectomycorrhizal (EM) tree species, yet the diversity of their EM fungal communities remains poorly explored. In lower montane forests in western Panama, the EM tree species Oreomunnea mexicana (Juglandaceae) forms locally dense populations in forest otherwise characterized by trees that form arbuscular mycorrhizal (AM) associations. The objective of this study was to compare the composition of EM fungal communities associated with Oreomunnea adults, saplings, and seedlings across sites differing in soil fertility and the amount and seasonality of rainfall. Analysis of fungal nrITS DNA (nuclear ribosomal internal transcribed spacers) revealed 115 EM fungi taxa from 234 EM root tips collected from adults, saplings, and seedlings in four sites. EM fungal communities were equally species-rich and diverse across Oreomunnea developmental stages and sites, regardless of soil conditions or rainfall patterns. However, ordination analysis revealed high compositional turnover between low and high fertility/rainfall sites located ca. 6 km apart. The EM fungal community was dominated by Russula (ca. 36 taxa). Cortinarius, represented by 14 species and previously reported to extract nitrogen from organic sources under low nitrogen availability, was found only in low fertility/high rainfall sites. Phylogenetic diversity analyses of Russula revealed greater evolutionary distance among taxa found on sites with contrasting fertility and rainfall than was expected by chance, suggesting that environmental differences among sites may be important in structuring EM fungal communities. More research is needed to evaluate whether EM fungal taxa associated with Oreomunnea form mycorrhizal networks that might account for local dominance of this tree species in otherwise diverse forest communities.






Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Andersen KM, Turner BL, Dalling JW (2010) Soil-based habitat partitioning in palms in lower montane tropical forests. J Biogeogr 37:278–292
Andersen KM, Endara MJ, Turner BL, Dalling JW (2012) Trait-based community assembly of understory palms along a soil nutrient gradient in a lower montane tropical forest. Oecologia 168:519–531
Arnold AE, Henk DA, Eells RL, Lutzoni F, Vilgalys R (2007) Diversity and phylogenetic affinities of foliar fungal endophytes in loblolly pine inferred by culturing and environmental PCR. Mycologia 99:185–206
Austin AT, Vitousek PM (1998) Nutrient dynamics on precipitation gradient in Hawai’i. Oecologia 113:519–529
Avis PG, McLaughlin DJ, Dentinger BC, Reich PB (2003) Long-term increase in nitrogen supply alters above- and below-ground ectomycorrhizal communities and increases the dominance of Russula spp. in a temperate oak savanna. New Phytol 160:239–253
Avis PG, Mueller GM, Lussenhop J (2008) Ectomycorrhizal fungal communities in two North American oak forests respond to nitrogen addition. New Phytol 179:472–483
Avis PG (2012) Ectomycorrhizal iconoclasts: the ITS rDNA diversity and nitrophilic tendencies of fetid Russula. Mycologia 104:998–1007
Bahram M, Põlme S, Kõljalg U, Tedersoo L (2011) A single European aspen (Populus tremula) tree individual may potentially harbor dozens of Cenococcum geophilum ITS genotypes and hundreds of species of ectomycorrhizal fungi. FEMS Microbiol Ecol 75:313–320
Bahram M, Põlme S, Kõljalg U, Zarre S, Tedersoo L (2012) Regional and local patterns of ectomycorrhizal fungal diversity and community structure along an altitudinal gradient in the Hyrcanian forests of northern Iran. New Phytol 193:465–473
Becker (1983) Ectomycorrhizae on Shorea (Dipterocarpaceae) seedlings in a lowland Malaysian rainforest. Malay For 46:146–170
Béreau M, Garbaye J (1994) First observations on the root morphology and symbioses of 21 major tree species in the primary tropical rain forest of French Guyana. Ann Sci For 51:407–416
Bergemann SE, Garbelotto M (2006) High diversity of fungi recovered from the roots of mature tanoak (Lithocarpus densiflorus) in northern California. Can J Bot 84:1380–1394
Bever JD, Platt TG, Morton ER (2012) Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu Rev Microbiol 66:265–83
Bonfante P, Genre A (2010) Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat comm 1:48
Booth MG, Hoeksema JD (2010) Mycorrhizal networks counteract competitive effects of canopy trees on seedling survival. Ecology 91:2294–2302
Buyck B, Hofstetter V, Eberhardt U, Verbeken A, Kauff F (2008) Walking the thin line between Russula and Lactarius: the dilemma of Russula subsect. Ochricompactae. Fungal Divers 28:15–40
Castresana J (2002) Gblocks server v. 0.91b, Institut de Biologia Evolutiva (CSIC-UPF). http://molevol.cmima.csic.es/castresana/Gblocks_server.html
Cavelier J (1996) Fog interception in montane forests across the central cordillera of Panama. J Trop Ecol 12:357–369
Connell JH, Lowman MD (1989) Low-diversity tropical rain forests: some possible mechanisms for their existence. Am Nat 134:88–119
Conway D, Alexander IJ (1992) Soil conditions under monodominant Gilbertiodendron dewevrei and mixed forest Ituri Forest Reserve, Zaire. Tropical Biology Newsletter 62:[unpaginated].
Courty PE, Franc A, Pierrat JC, Garbaye J (2008) Temporal changes in the ectomycorrhizal community in two soil horizons of a temperate oak forest. Appl Environ Microbiol 74:5792–5801
Dickie IA, Koide RT, Steiner KC (2002) Influences of established trees on mycorrhizas, nutrition, and growth of Quercus rubra seedlings. Ecol Monogr 72(4):505–521
Dickie IA, Bolstridge N, Cooper JA, Peltzer DA (2010) Co-invasion by Pinus and its mycorrhizal fungi. New Phytol 187:475–484
Diédhiou AG, Selosse MA, Galiana A, Diabate M, Dreyfus B, Ba AM, Miana de Faria S, Bena G (2010) Multi-host ectomycorrhizal fungi are predominant in a Guinean tropical rainforest and shared between canopy trees and seedlings. Environ Microbiol 12:2219–2232
Diédhiou AG, Christelle H, Ebenye M, Selosse MA, Onguene N, Ba AM (2014) Diversity and community structure of ectomycorrhizal fungi in mixed and monodominant African tropical rainforest. In: Bâ AM, McGuire KL, Diédhiou AG (eds) Ectomycorrhizal symbioses in tropical and neotropical forests. CRC Press, pp 1–18
Douglas RB, Parker VT, Cullings KW (2005) Belowground ectomycorrhizal community structure of mature lodgepole pine and mixed conifer stands in Yellowstone National Park. For Ecol Manag 208:303–317
Edgar R (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Egger KN, Hibbett DS (2004) The evolutionary implications of exploitation in mycorrhizas. Can J Bot 82:1110–1121
Ewing B, Green P (1998) Base-calling of automated sequencer traces using Phred. II. Error probabilities. Genome Res 8:186–194
Ewing B, Hillier L, Wendl M, Green P (1998) Base-calling of automated sequencer traces using Phred. I. Accuracy assessment. Genome Res 8:175–185
Faith DP (1992) Conservation evaluation and phylogenetic diversity. Biol Conserv 61:1–10
Gao Q, Yang ZL (2010) Ectomycorrhizal fungi associated with two species of Kobresia in an alpine meadow in the Western Himalaya. Mycorrhiza 20:281–287
Halling RE, Mueller GM (2005) Common mushrooms of the Talamanca Mountains. New York Botanical Garden Press, Bronx, NY, Costa Rica
Hart TB, Hart JA, Murphy PG (1989) Monodominant and species-rich forests in the humid tropics: causes for their co-occurrence. Am Nat 133:613–633
Henkel TW (2003) Monodominance in the ectomycorrhizal Dicymbe corymbosa (Caesalpiniaceae) from Guyana. J Trop Ecol 19:417–437
Henkel TW, Aime MC, Chin MML, Miller SL, Vilgalys R, Smith ME (2012) Ectomycorrhizal fungal sporocarp diversity and discovery of new taxa in Dicymbe monodominant forests of the Guiana Shield. Biodivers Conserv 21:2195–2220
Hughes KW, Petersen RH, Lickey EB (2009) Using heterozygosity to estimate a percentage DNA sequence similarity for environmental species’ delimitation across basidiomycete fungi. New Phytol 182:795–798
Ishida TA, Nara K, Hogetsu T (2007) Host effects on ectomycorrhizal fungal communities: insight from eight host species in mixed conifer-broadleaf forests. New Phytol 174:430–440
Itoh A (1995) Regeneration processes and coexistence mechanisms of two Bornean emergent dipterocarp species. Doctorate thesis, Kyoto University, Kyoto
Izzo A, Agbowo J, Bruns TD (2005) Detection of plot level changes in ectomycorrhizal communities across years in an old-growth mixed-conifer forest. New Phytol 166:619–629
Janos DP (1983) Tropical mycorrhizas, nutrient cycles and plant growth. In: Sutton SL, Whitmore TC, Chadwick AC (eds) Tropical rain forest: ecology and management. Blackwell Scientific Publications, Oxford, pp 327–345
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol 135:575–585
Jones MD, Twieg BD, Durall DM, Berch SM (2008) Location relative to a retention patch affects the ECM fungal community more than patch size in the first season after timber harvesting on Vancouver Island, British Columbia. For Ecol Manag 255:1342–1352
Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO (2010) Picante: R tools for integrating phylogenies and ecology. Bioinforma Appl Note 26:1463–1464
Kennedy PG, Izzo AD, Bruns TD (2003) There is high potential for the formation of common mycorrhizal networks between understory and canopy trees in a mixed evergreen forest. J Ecol 91:1071–1080
Kennedy PG, Smith DP, Horton TR, Molina R (2012) Arbutus menziesii (Ericaceae) facilitates regeneration dynamics in mixed evergreen forest by promoting mycorrhizal fungal diversity and host connectivity. Am J Bot 99:1691–1701
Kjøller R, Clemmensen KE (2009) Belowground ectomycorrhizal fungal communities respond to liming in three southern Swedish coniferous forest stands. For Ecol Manag 257:2217–2225
Kõljalg U et al (2013) Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 22:5271–5277
Krpata D, Peintner U, Langer I, Fitz WJ, Schweiger P (2008) Ectomycorrhizal communities associated with Populus tremula growing on a heavy metal contaminated site. Mycol Res 112:1069–1079
Lian C, Narimatsu M, Nara K, Hogetsu T (2006) Tricholoma matsutake in a natural Pinus densiflora forest: correspondence between above- and below-ground genets, association with multiple host trees and alteration of existing ectomycorrhizal communities. New Phytol 171:825–836
Lilleskov EA, Fahey TJ, Horton TR, Lovett GM (2002) Belowground ectomycorrhizal fungal community change over a nitrogen deposition gradient in Alaska. Ecology 83:104–115
Lozupone C, Hamady M, Knight R (2006) UniFrac—an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinformatics 7:371
Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R (2010) UniFrac: an effective distance metric for microbial community comparison. ISME J 5:169–72
Malloch DW, Pirozynski KA, Raven PH (1980) Ecological and evolutionary significance of mycorrhizal symbioses in vascular plants (A Review). Proc Natl Acad Sci 77:2113–2119
McGuire KL (2007) Common ectomycorrhizal networks may maintain monodominance in a tropical rain forest. Ecology 88:567–574
McGuire KL (2008) Ectomycorrhizal associations function to maintain tropical monodominance. In: Siddiqui ZA et al. (eds) Mycorrhizae: sustainable agriculture and forestry, Springer Science, pp 287–302
Miller SL, Buyck B (2002) Molecular phylogeny of the genus Russula in Europe with a comparison of modern infrageneric classifications. Mycol Res 106:259–276
Miller OK, Lodge DJ, Baroni TJ (2000) New and interesting ectomycorrhizal fungi from Puerto Rico, Mona, and Guana Islands. Mycologia 92:558–570
Morris MH, Smith ME, Rizzo DM, Rejmanek M, Bledsoe CS (2008) Contrasting ectomycorrhizal fungal communities on the roots of co-occurring oaks (Quercus spp.) in a California woodland. New Phytol 178:167–176
Morris MH, Pérez-Pérez MA, Smith ME, Bledsoe CS (2009) Influence of host species on ectomycorrhizal communities associated with two co-occurring oaks (Quercus spp.) in a tropical cloud forest. FEMS Microbiol Ecol 69:274–287
Moyersoen B (2006) Pakaraimaea dipterocarpacea is ectomycorrhizal, indicating an ancient Gondwanaland origin for the ectomycorrhizal habit in Dipterocarpaceae. New Phytol 172:753–762
Mueller GM, Halling RE, Carranza J, Mata M, Schmit JP (2006) Saprotrophic and ectomycorrhizal macrofungi of Costa Rican oak forests. In: Kappelle M (ed) Ecology and conservation of neotropical montane oak forests. Ecological Series 185. Springer, Heidelberg, pp 55–68
Mühlmann O, Peintner U (2008a) Ectomycorrhiza of Kobresia myosuroides at a primary successional glacier forefront. Mycorrhiza 18:355–362
Mühlmann O, Peintner U (2008b) Mycobionts of Salix herbacea on a glacier forefront in the Austrian Alps. Mycorrhiza 18:171–180
Mühlmann O, Bacher M, Peintner U (2008) Polygonum viviparum mycobionts on an alpine primary successional glacier forefront. Mycorrhiza 18:87–95
Nara K (2006) Pioneer dwarf willow may facilitate tree succession by providing late colonizers with compatible ectomycorrhizal fungi in a primary successional volcanic desert. New Phytol 171:187–198
O’Brien MJ, Gomola CE, Horton TR (2010) The effect of forest soil and community composition on ectomycorrhizal colonization and seedling growth. Plant Soil 341:321–331
Oksanen L, Kindt R, Legendre P, O’Hara B, Simpson GL, Solymos P, Henry M, Stevens H, Wagner H (2008) VEGAN: Community ecology package. R package version 1.15-1. http://cran.r-project.org/, http://vegan.r-forge.r-project.org/
Onguenea NA, Kuyper TW (2001) Mycorrhizal associations in the rain forest of South Cameroon. For Ecol Manag 140:277–287
Onguene NA, Kuyper TW (2002) Importance of the ectomycorrhizal network for seedling survival and ectomycorrhiza formation in rain forests of south Cameroon. Mycorrhiza 12:13–17
Palmer JM, Lindner DL, Volk TJ (2008) Ectomycorrhizal characterization of an American chestnut (Castanea dentata)-dominated community in Western Wisconsin. Mycorrhiza 19:27–36
Parrent JL, Vilgalys R (2007) Biomass and compositional responses of ectomycorrhizal fungal hyphae to elevated CO2 and nitrogen fertilization. New Phytol 176:164–174
Peay KG, Kennedy PG, Davies SJ, Tan S, Bruns TD (2010) Potential link between plant and fungal distributions in a dipterocarp rainforest: community and phylogenetic structure of tropical ectomycorrhizal fungi across a plant and soil ecotone. New Phytol 185:529–542
Peay KG, Kennedy PG, Bruns TD (2011) Rethinking ectomycorrhizal succession: are root density and hyphal exploration types drivers of spatial and temporal zonation? Fungal Ecol 4:233–240
Pena R, Offermann C, Simon J, Naumann PS, Gessler A, Holst J, Dannenmann M, Mayer H, Kögel-Knabner I, Rennenberg H, Polle A (2010) Girdling affects ectomycorrhizal fungal (EMF) diversity and reveals functional differences in EMF community composition in a beech forest. Appl Environ Microbiol 76:1831–1841
Peh KSH, Lewis SL, Lloyd J (2011) Mechanisms of monodominance in diverse tropical tree-dominated systems. J Ecol 99:891–898
Phosri C, Põlme S, Taylor AFS, Kõljalg U, Suwannasai N, Tedersoo L (2012) Diversity and community composition of ectomycorrhizal fungi in a dry deciduous dipterocarp forest in Thailand. Biodivers Conserv 21:2287–2298
Plamboeck AH, Dawson TE, Egerton-Warburton LM, North M, Bruns TD, Querejeta JI (2007) Water transfer via ectomycorrhizal fungal hyphae to conifer seedlings. Mycorrhiza 17:439–447
Quist D, Garbelotto M, Chapela IH (1999) Mycorrhizal ecology of Oreomunnea: implications of fungal community structure on plant distribution and diversity. Preliminary investigations in the Sierra Juarez, Oaxaca, Mexico. Abstract In: Libro de resúmenes del III Congreso Latinoamericano de Micología, pp 99–100
R Development Core Team (2011) R: a language and environment for statistical computing. In: R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
Richard F, Millot S, Gardes M, Selosse M-A (2005) Diversity and specificity of ectomycorrhizal fungi retrieved from an old-growth Mediterranean forest dominated by Quercus ilex. New Phytol 166:1011–1023
Richard F, Roy M, Shahin O, Sthultz C, Duchemin M, Joffre R, Selosse M-A (2011) Ectomycorrhizal communities in a Mediterranean forest ecosystem dominated by Quercus ilex: seasonal dynamics and response to drought in the surface organic horizon. Ann For Sci 68:57–68
Simard SW, Perry DA, Jones MD, Myrold DD, Durall DM, Molina R (1997) Net transfer of carbon between tree species with shared ectomycorrhizal fungi. Nature 388:579–582
Simard SW, Beiler KJ, Bingham MA, Deslippe JR, Philip LJ, Teste FP (2012) Mycorrhizal networks: mechanisms, ecology and modeling. Fungal Biol Rev 26:39–60
Roy M, Rochet J, Manzi S, Jargeat P, Gryta H, Moreau P-A, Gardes M (2013) What determines Alnus-associated ectomycorrhizal community diversity and specificity? A comparison of host and habitat effects at a regional scale. New Phytol 198:1228–1238
Ryberg M, Larsson E, Molau U (2009) Ectomycorrhizal diversity in Dryas octopetala and Salix reticulata in an Alpine cliff ecosystem. Arct Alp Res 41:506–514
Ryberg M, Andreasen M, Björk RG (2010) Weak habitat specificity in ectomycorrhizal communities associated with Salix herbacea and Salix polaris in alpine tundra. Mycorrhiza 21:289–296
Smith EP, van Belle G (1984) Nonparametric estimation of species richness. Biometrics 40:119–129
Smith JE, McKay D, Niwa CG, Thies WG, Brenner G, Spatafora JW (2004) Short-term effects of seasonal prescribed burning on the ectomycorrhizal fungal community and fine root biomass in ponderosa pine stands in the Blue Mountains of Oregon. Can J For Res 34:2477–2491
Smith JE, McKay D, Brenner G, McIver J, Spatafora JW (2005) Early impacts of forest restoration treatments on the ectomycorrhizal fungal community and fine root biomass in a mixed conifer forest. J Appl Ecol 42:526–535
Smith ME, Douhan GW, Rizzo DM (2007a) Intra-specific and intra-sporocarp ITS variation of ectomycorrhizal fungi as assessed by rDNA sequencing of sporocarps and pooled ectomycorrhizal roots from a Quercus woodland. Mycorrhiza 18:15–22
Smith ME, Douhan GW, Rizzo DM (2007b) Ectomycorrhizal community structure in a xeric Quercus woodland based on rDNA sequence analysis of sporocarps and pooled roots. New Phytol 174:847–863
Smith ME, Douhan GW, Fremier AK, Rizzo DM (2009) Are true multihost fungi the exception or the rule? Dominant ectomycorrhizal fungi on Pinus sabiniana differ from those on co-occurring Quercus species. New Phytol 182:295–299
Smith ME, Henkel TW, Aime MC, Fremier AK, Vilgalys R (2011) Ectomycorrhizal fungal diversity and community structure on three co-occurring leguminous canopy tree species in a Neotropical rainforest. New Phytol 192:699–712
Smith ME, Henkel TW, Uehling JK, Fremier AK, Clarke HD, Vilgalys R (2013) The ectomycorrhizal fungal community in a neotropical forest dominated by the endemic dipterocarp Pakaraimea dipterocarpacea. PLoS ONE 8, e55160
Smith SE, Read DJ (2008) Introduction. In: Smith SE, Read DJ (eds) Mycorrhizal symbiosis 3rd edn. Academic Press
St John TV (1980) A survey of mycorrhizal infection in an Amazonian rain forest. Acta Amazon 10:527–533
St John TV, Uhl C (1983) Mycorrhizae in the rainforest at San Carlos de Rio Negro, Venezuela. Acta Cient Venez 34:233–237
Stone DE (1972) New world juglandaceae, III. A new perspective of the tropical members with winged fruits. Ann. Mo. Bot. Gard. 59:297–322
Taniguchi T, Kanzaki N, Tamai S, Yamanaka N, Futai K (2007) Does ectomycorrhizal fungal community structure vary along a Japanese black pine (Pinus thunbergii) to black locust (Robinia pseudoacacia) gradient? New Phytol 173:322–334
Taylor AFS, Martin F, Read DJ (2000) Fungal diversity in ectomycorrhizal communities of Norway spruce [Picea abies (L.) Karst.] and beech (Fagus sylvatica L.) along north–south transects in Europe. In: Detlef Schulze ED (ed) Carbon and nitrogen cycling in European forest ecosystems. Ecological Studies v. 142. Springer, Heidelberg, pp 343–365
Tedersoo L, Kõljalg U, Hallenberg N, Larsson K-H (2003) Fine scale distribution of ectomycorrhizal fungi and roots across substrate layers including coarse woody debris in a mixed forest. New Phytol 159:153–165
Tedersoo L, Suvi T, Larsson E, Kõljalg U (2006) Diversity and community structure of ectomycorrhizal fungi in a wooded meadow. Mycol Res 110:734–748
Tedersoo L, Suvi T, Beaver K, Kõljalg U (2007) Ectomycorrhizal fungi of the Seychelles: diversity patterns and host shifts from the native Vateriopsis seychellarum (Dipterocarpaceae) and Intsia bijuga (Caesalpiniaceae) to the introduced Eucalyptus robusta (Myrtaceae), but not Pinus caribea (Pinaceae). New Phytol 175:321–333
Tedersoo L, Jairus T, Horton B, Abarenkov K, Suvi T, Saar I, Kõljalg U (2008) Strong host preference of ectomycorrhizal fungi in a Tasmanian wet sclerophyll forest as revealed by DNA barcoding and taxon-specific primers. New Phytol 180:479–490
Tedersoo L, Sadam A, Zambrano M, Valencia R (2010a) Low diversity and high host preference of ectomycorrhizal fungi in Western Amazonia, a Neotropical biodiversity hotspot. ISME J 4:465–471
Tedersoo L, Way TW, Smith ME (2010b) Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20:217–263
Tedersoo L, Bahram M, Jairus T, Bechem E, Chinoya S, Mpumba R, Leal M, Randrianjohany E, Razafimandimbison S, Sadam A, Naadel T, Koljalg U (2011) Spatial structure and the effects of host and soil environments on communities of ectomycorrhizal fungi in wooded savannas and rain forests of Continental Africa and Madagascar. Mol Ecol 20:3071–3080
Tedersoo L, Bahram M, Toots M, Diédhiou AG, Henkel TL, Kjoller R, Morris MH, Nara K, Nouhara E, Peay K, Polme S, Ryberg M, Smith ME, Koljalg U (2012) Towards global patterns in the diversity and community structure of ectomycorrhizal fungi. Mol Ecol 21:4160–4170
Ter Braak CJF (1995) Ordination. In: Jongman RHG, Ter Braak CJF, Van Tongeren OFR (eds) Data analysis in community and landscape ecology. Cambridge University Press, New York, pp 91–173
Teste FP, Simard SW, Durall DM, Guy RD, Jones MD, Schoonmaker AL (2009) Access to mycorrhizal networks and roots of trees: importance for seedling survival and resource transfer. Ecology 90:2808–2822
Toljander JF, Eberhardt U, Toljander YK, Paul LR, Taylor AFS (2006) Species composition of an ectomycorrhizal fungal community along a local nutrient gradient in a boreal forest. New Phytol 170:873–883
Twieg B, Durall DM, Simard SW (2007) Ectomycorrhizal fungal succession in mixed temperate forests. New Phytol 176:437–447
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355
U’Ren JM, Dalling JW, Gallery RE, Maddison DR, Davis EC, Gibson CM, Arnold AE (2009) Diversity and evolutionary origins of fungi associated with seeds of a Neotropical pioneer tree: a case study for analysing fungal environmental samples. Mycol Res 113:432–449
U’Ren JM, Lutzoni F, Miadlikowska J, Laetsch AD, Arnold AE (2012) Host and geographic structure of endophytic and endolichenic fungi at a continental scale. Am J Bot 99:898–914
Walker JF, Miller OK, Horton JL (2005) Hyperdiversity of ectomycorrhizal fungus assemblages on oak seedlings in mixed forests in the Southern Appalachian Mountains. Mol Ecol 14:829–838
Zwickl DJ (2006) Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. Ph.D. dissertation, The University of Texas at Austin
Acknowledgments
Funding from a Tinker Summer Research Fellowship, a Francis M. and Harlie M. Clark Research support grant, a Robert L. Gilbertson Mycological Herbarium Grant (University of Arizona), the College of Agriculture and Life Sciences at The University of Arizona, and the Smithsonian Tropical Research Institute Short-Term Fellowship program is gratefully acknowledged. We sincerely thank two anonymous reviewers whose comments greatly improved the manuscript. Forest plot work was supported by grant COLO08-003 from the Government of Panama (SENACYT). We thank Kayla Arendt, Jana U’Ren, Katy Heath, and Pat Burke for assistance with molecular work, and Katie Heineman, Carmen Velasquez, Carlos Espinosa, and Marggie Rodriguez for their help in the field.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Results of maximum likelihood analysis of Russula obtained from mycorrhizal root tips (by direct PCR) and fruiting bodies in Oreomunnea mexicana-dominated stands at Fortuna, Panama (bold font) and exemplar taxa chosen as described in the text. Thickened branches indicate ≥70 % bootstrap support. Strains from root tips are annotated to indicate the life stage (seedling, sapling, adult), site (low fertility/high rainfall in blue font—HA Honda A, HB Honda B; high fertility/low rainfall in green font—HO Hornito, AF Alto Frio), name and accession number for the closest match to the sequence in GenBank, and sequencing code. Sequences from fruit bodies are annotated to indicate site (those listed previously; collections also were made at ZA, Zarceadero; see supplementary material), name and accession number for the closest match to the sequence in GenBank, and sequencing code (PDF 89 kb)
Table S1
(XLSX 78 kb)
Table S2
(XLSX 47 kb)
Rights and permissions
About this article
Cite this article
Corrales, A., Arnold, A.E., Ferrer, A. et al. Variation in ectomycorrhizal fungal communities associated with Oreomunnea mexicana (Juglandaceae) in a Neotropical montane forest. Mycorrhiza 26, 1–17 (2016). https://doi.org/10.1007/s00572-015-0641-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-015-0641-8