Abstract
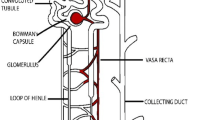
Kidney failure is a common disease prevailing in the present generation. Renal disfunction inflict adverse effects on human health that are much fatal. Kidney failure has attracted great attention among the present scientific community. Thus, it is desirable to develop an implantable device to replace the failed kidney that could be partially possible with Kidney-on-Chip technology. This paper presents a Kidney-on-Chip model where size-dependent re-absorption of the kidney is majorly investigated by considering the implications of hydrostatic pressure. The device is comprised of two blood tubules separated by a main tubule in the Centre. The main tubule connects both the blood tubules by the means of transporting channels. The transporting channel allows the flow of solutes from the main tubule to blood tubules depending on size of the solutes. The hydrostatic effect can alter the functioning of the kidney by effecting the cytoskeleton arrangement of kidney cells. Employing hydrostatic effects into the device will make the model more compact and realistic. The analysis was made by employing hindrance in the form of bars arranged in zig–zag fashion in the main tubule forming stepwise structure. Simulation results of presented design have produced an outflow velocity of 9.12 × 10−5 m/s which is greater than 8.2 × 10−5 m/s without hydrostatic effect. The re-absorption rate has been improved from 50 to 55% which is desirable. The mathematical analysis was made to support the simulation results. Different studies were conducted on the device performance by changing the parameters that affect fluid flow such as inflow velocity, density, viscosity, capillary forces, and surface tension. The presented work can produce a bio-reactor for kidney-on-Chip applications with more realistic results.









Similar content being viewed by others
References
Al-Awqati Q (2012) Basic research in nephrology: Are we in decline? J Am Soc Nephrol 23(10):1611–1616
Couser WG, Remuzzi G, Mendis S, Tonelli M (2011) The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 80(12):1258–1270
Frohlich EM, Zhang X, Charest JL (2011) The use of controlled surface topography and flow-induced shear stress to influence renal epithelial cell function. Integr Biol 4(1):75–83
Goldman L, Schafer A (2011) Goldman’s cecil medicine E-book. Elsevier Health Sciences, Amsterdam
Huang HC, Chang YJ, Chen WC, Harn HIC, Tang MJ, Wu CC (2013) Enhancement of renal epithelial cell functions through microfluidic-based coculture with adipose-derived stem cells. Tissue Eng Part A 19(17–18):2024–2034
Jang KJ, Mehr AP, Hamilton GA, McPartlin LA, Chung S, Suh KY, Ingber DE (2013) Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol 5(9):1119–1129
Lang Q, Ren Y, Wu Y, Guo Y, Zhao X, Tao Y, Jiang H (2016) A multifunctional resealable perfusion chip for cell culture and tissue engineering. RSC Adv 6(32):27183–27190
Li X, Valadez AV, Zuo P, Nie Z (2012) Microfluidic 3D cell culture: potential application for tissue-based bioassays. Bioanalysis 4(12):1509–1525
Li Z, Jiang L, Tao T, Su W, Guo Y, Yu H, Qin J (2017a) Assessment of cadmium-induced nephrotoxicity using a kidney-on-a-chip device. Toxicol Res 6(3):372–380
Li Z, Su W, Zhu Y, Tao T, Li D, Peng X, Qin J (2017b) Drug absorption related nephrotoxicity assessment on an intestine-kidney chip. Biomicrofluidics 11(3):034114
Long KR, Shipman KE, Rbaibi Y, Menshikova EV, Ritov VB, Eshbach ML, Weisz OA (2017) Proximal tubule apical endocytosis is modulated by fluid shear stress via an mTOR-dependent pathway. Mol Biol Cell 28(19):2508–2517
Maschmeyer I, Lorenz AK, Schimek K, Hasenberg T, Ramme AP, Hübner J, Sambo NS (2015) A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 15(12):2688–2699
Ohno A, Beck FX, Pfaller W, Giebisch G, Wang T (1995) Effects of chronic hyperfiltration on proximal tubule bicarbonate transport and cell electrolytes. Kidney Int 48(3):712–721
Oo ZY, Deng R, Hu M, Ni M, Kandasamy K, Bin Ibrahim MS, Zink D (2011) The performance of primary human renal cells in hollow fiber bioreactors for bioartificial kidneys. Biomaterials 32(34):8806–8815
Quinton W, Dillard D, Scribner BH (1960) Cannulation of blood vessels for prolonged hemodialysis. ASAIO J 6(1):104–113
Sateesh J, Guha K, Dutta A, Sengupta P, Rao KS (2019a) Design and analysis of microfluidic kidney-on-chip model: fluid shear stress based study with temperature effect. Microsyst Technol 25(7):2553–2560
Sateesh J, Guha K, Dutta A, Sengupta P, Rao KS (2019b). Mimicking proximal tubule cell functioning for artificial kidney applications. Technconnect Briefs, pp 306–309
Snouber LC, Jacques S, Monge M, Legallais C, Leclerc E (2012) Transcriptomic analysis of the effect of ifosfamide on MDCK cells cultivated in microfluidic biochips. Genomics 100(1):27–34
Stanifer JW (2018) The global burden of kidney disease and the sustainable development goals. Bulletin of the World Health Organization
Wilmer MJ, Ng CP, Lanz HL, Vulto P, Suter-Dick L, Masereeuw R (2016) Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol 34(2):156–170
Woodrow G, Turney JH, Brownjohn AM (1997) Technique failure in peritoneal dialysis and its impact on patient survival. Perit Dial Int 17(4):360–364
Yao W, Hu Q, Ma Y, Xiong W, Wu T, Cao J, Wu D (2015) Human adipose-derived mesenchymal stem cells repair cisplatin-induced acute kidney injury through antiapoptotic pathways. Exp Ther Med 10(2):468–476
Yeung CK, Himmelfarb J (2019) Kidneys on chips: emerging technology for preclinical drug development. Clin J Am Soc Nephrol 14(1):144–146
Zuo P, Li X, Dominguez DC, Ye BC (2013) A PDMS/paper/glass hybrid microfluidic biochip integrated with aptamer-functionalized graphene oxide nano-biosensors for one-step multiplexed pathogen detection. Lab Chip 13(19):3921–3928
Acknowledgements
The authors would like to heartfully thank Ms. M K S L Gayatri for her invaluable efforts in analytical modelling of the fluid dynamics and suggestion in design part. Further, the authors also thankful to NMDC NIT Silchar for providing the necessary financial support to carryout the project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guha, K., Sateesh, J., Dutta, A. et al. Mimicking kidney re-absorption using microfluidics by considering hydrostatic pressure inside kidney tubules: structural and analytical study. Microsyst Technol 26, 1769–1776 (2020). https://doi.org/10.1007/s00542-019-04720-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00542-019-04720-9