Abstract
Introduction
The gold standard for measuring the partial pressure of carbon dioxide remains arterial blood gas (ABG) analysis. For patients with cystic fibrosis undergoing general anesthesia or polysomnography studies, continuous non-invasive carbon dioxide monitoring may be required. The current study compares end-tidal (ETCO2), transcutaneous (TCCO2), and capillary blood gas carbon dioxide (Cap-CO2) monitoring with the partial pressure of carbon dioxide (PaCO2) from an ABG in patients with cystic fibrosis.
Methods
Intraoperatively, a single CO2 value was simultaneously obtained using ABG (PaCO2), capillary (Cap-CO2), TCCO2, and ETCO2 techniques. Tests for correlation (Pearson’s coefficient) and agreement (Bland–Altman analysis) were performed. Data were further stratified into two subgroups based on body mass index (BMI) and percent predicted forced expiratory volume in 1 s (FEV1%). Additionally, the absolute difference in the TCCO2, ETCO2, and Cap-CO2 values versus PaCO2 was calculated. The mean ± SD differences were compared using a paired t test while the number of times the values were ≤ 3 mmHg and ≤ 5 mmHg from the PaCO2 were compared using a Fishers’ exact test.
Results
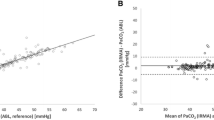
The study cohort included 47 patients (22 males, 47%) with a mean age of 13.4 ± 7.8 years, median (IQR) BMI of 18.7 kg/m2 (16.7, 21.4), and mean FEV1% of 87.3 ± 18.3%. Bias (SD) was 4.8 (5.7) mmHg with Cap-CO2 monitoring, 7.3 (9.7) mmHg with TCCO2 monitoring, and 9.7 (7.7) mmHg with ETCO2 monitoring. Although there was no difference between the degree of bias in the population as a whole, when divided based on FEV1% and BMI, there was greater bias with ETCO2 in patients with a lower FEV1% and a higher BMI. The Cap-CO2 vs. PaCO2 difference was 5.2 ± 5.3 mmHg (SD), with 16 (48%) ≤ 3 mmHg and 20 (61%) ≤ 5 mmHg from the ABG value. The TCCO2–PaCO2 difference was 9.1 ± 7.2 mmHg (SD), with 11 (27%) ≤ 3 mmHg and 15 (37%) ≤ 5 mmHg from the ABG value. The ETCO2–PaCO2 mean difference was 11.2 ± 7.9 mmHg (SD), with 5 (12%) ≤ 3 mmHg and 11 (26%) ≤ 5 mmHg from the ABG value.
Conclusions
While Cap-CO2 most accurately reflects PaCO2 as measured on ABG, of the non-invasive continuous monitors, TCCO2 was a more accurate and reliable measure of PaCO2 than ETCO2, especially in patients with worsening pulmonary function (FEV1% ≤ 81%) and/or a higher BMI (≥ 18.7 kg/m2).
Similar content being viewed by others
References
Elborn JS. Cystic fibrosis. Lancet. 2016;388:2519–31.
Bhavani-Shankar K, Moseley H, Kumar AY. Capnometry and anaesthesia. Can J Anaesth. 1992;39:617–32.
Bhende M. Capnography in the pediatric emergency department. Pediatr Emerg Care. 1999;15:64–9.
Pansard JL, Cholley B, Devilliers C, et al. Variation in the arterial to end-tidal CO2 tension differences during anesthesia in the “kidney rest” lateral decubitus position. Anesth Analg. 1992;75:506–10.
Grenier B, Verchere E, Meslie A, et al. Capnography monitoring during neurosurgery: reliability in relation to various intraoperative positions. Anesthesiology. 1999;88:43–8.
Short JA, Paris ST, Booker BD, et al. Arterial to end-tidal carbon dioxide tension difference in children with congenital heart disease. Br J Anaesth. 2001;86:349–53.
Burrows FA. Physiologic dead space, venous admixture and the arterial to end-tidal carbon dioxide difference in infants and children undergoing cardiac surgery. Anesthesiology. 1989;70:219–25.
Badgwell JM, Heavener JE, May WS, et al. End-tidal PCO2 monitoring in infants and children ventilated with either a partial rebreathing or non-rebreathing circuit. Anesthesiology. 1987;66:405–10.
Badgwell JM, McLeod ME, Lerman J, et al. End-tidal PCO2 measurements sampled at the distal and proximal ends of the endotracheal tube in infants and children. Anesth Analg. 1987;66:959–64.
Tobias JD. Transcutaneous carbon dioxide monitoring in infants and children. Paediatr Anaesth. 2009;19:434–44.
Cox P, Tobias J. Noninvasive monitoring of PaCO2 during one-lung ventilation and minimal access surgery in adults: end-tidal versus transcutaneous techniques. J Min Access Surg. 2007;3:8–13.
Tobias J. Noninvasive carbon dioxide monitoring during one-lung ventilation: end-tidal versus transcutaneous techniques. J Cardiothor Vasc Anesth. 2003;17:306–8.
Wilson J, Russo P, Russo J, Tobias J. Noninvasive monitoring of carbon dioxide in infants and children with congenital heart disease: end-tidal versus transcutaneous techniques. J Intensive Care Med. 2005;20:291–5.
Griffin J, Terry BE, Burton RK, Ray TI, Keller BP, Landrum AL, et al. Comparison of end-tidal and transcutaneous measures of carbon dioxide during general anesthesia in severely obese adults. Br J Anaesthesia. 2003;91:498–501.
Domingo C, Canturri E, Lujan M, Moreno A, Espuelas H, Marin A. Transcutaneous measurement of partial pressure of carbon dioxide and oxygen saturation: validation of the SenTec monitor. Arch Bronconeumol. 2006;42:246–51.
Yildizdas D, Yapicioglu H, Yilmaz HL, Sertdemir Y. Correlation of simultaneously obtained capillary, venous and arterial blood gases of patients in a pediatric intensive care unit. Arch Dis Child. 2004;89:176–80.
Escalante-Kanashiro R, Tantalean-Da-Fieno J. Capillary blood gases in a pediatric intensive care unit. Crit Care Med. 2000;28:224–6.
Zavorsky GS, Cao J, Mayo NE, Gabbay R, Murias JM. Arterial versus capillary blood gases: a meta-analysis. Resp Phys Neurobiol. 2007;155:268–79.
Harrison MA, Lynch JM, Dean MJ, Witte MK. Comparison of simultaneously obtained arterial and capillary blood gases in pediatric intensive care unit patients. Crit Care Med. 1997;25:1904–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
May, A., Humston, C., Rice, J. et al. Non-invasive carbon dioxide monitoring in patients with cystic fibrosis during general anesthesia: end-tidal versus transcutaneous techniques. J Anesth 34, 66–71 (2020). https://doi.org/10.1007/s00540-019-02706-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-019-02706-5