Abstract
Purpose
Malignant hyperthermia (MH) is an inherited muscle disorder caused by abnormal elevations of intracellular calcium (Ca2+) in skeletal muscle. There are several reports of myotoxicity caused by local anesthetics, and the increased intracellular Ca2+ is considered to be an important cause. However, there is insufficient evidence regarding myotoxicity in MH-susceptible individuals when large doses of local anesthetics are administered. This study investigated the effect of MH predisposition on myotoxicity.
Methods
Human skeletal muscle samples were obtained from 22 individuals to determine susceptibility to MH, and were evaluated according to whether their Ca2+-induced Ca2+ release (CICR) rates were accelerated or not. This study was performed using surplus muscle that remained after the CICR rate test. We calculated the 50% effective concentration (EC50) values of three local anesthetics, namely lidocaine, levobupivacaine, and ropivacaine using the ratiometric dye Fura-2 AM. Significance was tested using the unpaired t test.
Results
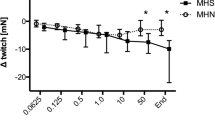
In the accelerated and unaccelerated groups, respectively, the mean ± SD of the EC50 values were 1.52 ± 0.72 and 1.75 ± 0.37 mM for lidocaine (p = 0.42), 0.72 ± 0.36 and 0.79 ± 0.46 mM for levobupivacaine (p = 0.68), and 1.21 ± 0.35 and 1.62 ± 0.57 mM for ropivacaine (p = 0.06). These values were similar in individuals with and without MH predisposition.
Conclusion
The myotoxicity of local anesthetics was equivalent in individuals with and without predisposition to MH.





Similar content being viewed by others
References
Halsall PJ, Ellis FR. Malignant hyperthermia. Anaesth Intensive Care. 2005;14:192–4.
Schneiderbanger D, Johannsen S, Roewer N, Schuster F. Management of malignant hyperthermia: diagnosis and treatment. Ther Clin Risk Manag. 2014;10:355–62.
Zink W, Bohl JRE, Hacke N, Sinner B, Martin E, Graf BM. The long term myotoxic effects of bupivacaine and ropivacaine after continuous peripheral nerve blocks. Anesth Analg. 2005;101:548–54.
Neal JM, Salinas FV, Choi DS. Local anesthetic-induced myotoxicity after continuous adductor canal block. Reg Anesth Pain Med. 2016;41:723–7.
Benoit PW, Yagiela A, Fort NF. Pharmacologic correlation between local anesthetic-induced myotoxicity and disturbances of intracellular calcium distribution. Toxicol Appl Pharmacol. 1980;52:187–98.
Plank C, Hofmann P, Gruber M, Bollwein G, Graf BM, Zink W, et al. Modification of bupivacaine-induced myotoxicity with dantrolene and caffeine in vitro. Anesth Analg. 2016;122:418–23.
Harrison GG, Morrell DF. Response of mhs swine to i.v. infusion of lignocaine and bupivacaine. Br J Anaesth. 1980;52:385–7.
Wingard DW, Bobko S. Failure of lidocaine to trigger porcine malignant hyperthermia. Anesth Analg. 1979;58:99–103.
Ibarra MCA, Ichihara Y, Hikita M, Yoshida K, Junji S, Maehara Y, et al. Effect of bupivacaine enantiomers on Ca2+ release from sarcoplasmic reticulum in skeletal muscle. Eur J Pharmacol. 2005;512:77–83.
Maemura Y. Effect of ropivacaine on Ca function of skinned skeletal muscle. Masui. 2002;51:19–24.
Endo M, Iino M. Measurement of Ca2 + release in skinned fibers from skeletal muscle. Methods Enzymol. 1988;157:12–26.
Ohta T, Endo M, Nakano T, Morohoshi Y, Wanikawa K, Ohga A. Ca-induced Ca release in malignant hyperthermia-susceptible pig skeletal muscle. Am J Physiol. 1989;256:C358–67.
Oku S, Mukaida K, Nosaka S, Sai Y, Maehara Y, Yuge O. Comparison of the in vitro caffeine-halothane contracture test with the Ca-induced Ca release rate test in patients suspected of having malignant hyperthermia susceptibility. J Anesth. 2000;14:6–13.
Kobayashi M, Mukaida K, Migita T, Hamada H, Kawamoto M, Yuge O. Analysis of human cultured myotubes responses mediated by ryanodine receptor 1. Anaesth Intensive Care. 2011;39:252–61.
Hofmann P, Metterlein T, Bollwein G, Gruber M, Plank C, Graf BM, et al. The myotoxic effect of bupivacaine and ropivacaine on myotubes in primary mouse cell culture and an immortalized cell line. Anesth Analg. 2013;117:634–40.
Zink W, Seif C, Bohl JRE, Hacke N, Braun PM, Sinner B, et al. The acute myotoxic effects of bupivacaine and ropivacaine after continuous peripheral nerve blockades. Anesth Analg. 2003;97:1173–9 (table of contents).
Zhang C, Phamonvaechavan P, Rajan A, Poon DY, Topcu-Yilmaz P, Guyton DL. Concentration-dependent bupivacaine myotoxicity in rabbit extraocular muscle. J AAPOS. 2010;14:323–7.
Benoit PW, Belt WD. Destruction and regeneration of skeletal muscle after treatment with a local anaesthetic, bupivacaine (Marcaine). J Anat. 1970;107:547–56.
McLoon LK, Nguyen LT, Wirtschafter J. Time course of the regenerative response in bupivacaine injured orbicularis oculi muscle. Cell Tissue Res. 1998;294:439–47.
Zink W, Graf BM. Local anesthetic myotoxicity. Reg Anesth Pain Med.;29:333–40.
Melzer W, Herrmann-Frank A, Lüttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta. 1995;1241:59–116.
Schiemann AH, Stowell KM. Comparison of pathogenicity prediction tools on missense variants in RYR1 and CACNA1S associated with malignant hyperthermia. Br J Anaesth. 2016;117:124–8.
Shoshan-Barmatz V, Zchut S. The interaction of local anesthetics with the ryanodine receptor of the sarcoplasmic reticulum. J Membr Biol. 1993;133:171–81.
Hyvelin JM, Martin C, Roux E, Marthan R, Savineau JP. Human isolated bronchial smooth muscle contains functional ryanodine/caffeine-sensitive Ca-release channels. Am J Respir Crit Care Med. 2000;162:687–94.
Gordienko DV, Harhun MI, Kustov MV, Pucovský V, Bolton TB. Sub-plasmalemmal [Ca2+]i upstroke in myocytes of the guinea-pig small intestine evoked by muscarinic stimulation: IP3R-mediated Ca2+ release induced by voltage-gated Ca2+ entry. Cell Calcium. 2008;43:122–41.
Prakriya M, Lewis RS. Store-operated calcium channels. Physiol Rev. 2015;95:1383–436.
Franco-Obregón A, Lansman JB. Changes in mechanosensitive channel gating following mechanical stimulation in skeletal muscle myotubes from the mdx mouse. J Physiol. 2002;539:391–407.
Riazi S, Kraeva N, Muldoon SM, Dowling J, Ho C, Petre M-A, et al. Malignant hyperthermia and the clinical significance of type-1 ryanodine receptor gene (RYR1) variants: proceedings of the 2013 MHAUS Scientific Conference. Can J Anesth. 2014;61:1040–9.
Hopkins PM, Rüffert H, Snoeck MM, Girard T, Glahn KPE, Ellis FR, et al. European Malignant Hyperthermia Group guidelines for investigation of malignant hyperthermia susceptibility. Br J Anaesth. 2015;115:531–9.
Wehner M, Rueffert H, Koenig F, Meinecke CD, Olthoff D. The Ile2453Thr mutation in the ryanodine receptor gene 1 is associated with facilitated calcium release from sarcoplasmic reticulum by 4-chloro-m-cresol in human myotubes. Cell Calcium. 2003;34:163–8.
Acknowledgements
A Grant-in-Aid (Grant Number 16K20100) for Scientific Research from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
About this article
Cite this article
Otsuki, S., Yasuda, T., Mukaida, K. et al. Myotoxicity of local anesthetics is equivalent in individuals with and without predisposition to malignant hyperthermia. J Anesth 32, 616–623 (2018). https://doi.org/10.1007/s00540-018-2526-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-018-2526-4