Abstract
Purpose
Recent clinical practice guidelines have recommended ambulatory management of febrile neutropenia in patients with low risk of complications. Although some centers have begun developing management protocols for these patients, there appears to be a certain reluctance to implement them in clinical practice. Our aim is to evaluate the strengths and weaknesses of this strategy according to available evidence and to propose new lines of research.
Methods
Systematic review using a triple aim approach (efficacy, cost-effectiveness, and quality of life), drawing from literature in MEDLINE (PubMed), Embase, and Cochrane Library databases. The review includes studies that assess ambulatory management for efficacy, cost-efficiency, and quality of life.
Results
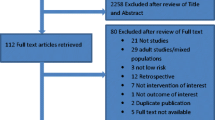
The search yielded 27 articles that met our inclusion criteria.
Conclusion
In conclusion, based on current evidence, ambulatory management of febrile neutropenia is safe, more cost-effective than inpatient care, and capable of improving quality of life in oncological patients with this complication. Ambulatory care seems to be an effective alternative to hospitalization in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neutropenia is a risk factor for certain infections that may appear as a frequent and severe complication in oncological patients; its only typical symptom is fever [1].
This entity has classically been treated through hospitalized management and intravenous (i.v.) administration of wide-spectrum antibiotics in all patients. Currently, only a fraction of patients with febrile neutropenia present serious complications, and there is evidence of a diverse prognostic spectrum [2,3,4,5,6,7,8,9,10]. In light of this fact, the main scientific societies at a national and international level, including the American Society for Clinical Oncology (ASCO) [11] and the Spanish Society for Medical Oncology (SEOM) [12], make a distinction between patients with febrile neutropenia who are at high or low risk, depending on defined criteria set out in globally validated scales like the ones from the Talcott group [3], the Multinational Association of Supportive Care in Cancer (MASCC) [13], and the clinical index of stable febrile neutropenia (CISNE) [14]. Better understanding of these different risk profiles, plus the emergence of new care models like home hospital care, have raised the possibility of less aggressive treatment options, for instance simplified oral monotherapy and immediate or early discharge following an observation period.
This qualitative systematic review aimed to assess ambulatory care strategies in adult patients with febrile neutropenia through a triple aim, examining efficacy, cost-effectiveness, and quality of life. These results can inform the definition of the most adequate setting for managing these patients (ambulatory care, home hospitalization, or inpatient care); contribute to updating the action protocols in emergency services; and identify areas where there is uncertainty in the available evidence in order to trace new lines of research.
Methods
Design and search strategy
Using the MEDLINE (PubMed), Embase, and Cochrane Library databases, a search was undertaken of all meta-analyses, clinical trials, and observational studies published up to February 2021. We also performed backward reference searching on the bibliographies of the most recent ASCO [11] and SEOM [12] clinical guidelines to look for additional studies that met our inclusion criteria.
The search strategy used the following terms:
“Febrile neutropenia,” “low risk febrile neutropenia,” and “Low risk Neutropenic fever” in three search strings, each combined with the following terms using the Boolean operator AND: “Hospital admission,” “inpatient management,” “outpatient management,” “Home admission,” “Ambulatory management,” “Early discharge,” “immediate discharge,” “delay discharge,” “Observation before discharge,” and “outpatient treatment.”
Study selection
Eligible studies were those comparing in-hospital versus ambulatory management with immediate or early discharge, those assessing simple or combined treatment regimens, and those aiming to define the most appropriate administration route. We also included studies that analyzed the costs of different interventions or evaluated patient-reported quality of life according to the applied treatment strategy.
Studies in pediatric or adolescent populations (< 18 years of age) were excluded, as oncological pathologies have peculiarities in this group that are not generalizable to adults.
Results
The results of the search and the study flow chart are presented in Fig. 1.
Description of included studies
The criteria for defining the concepts of fever and neutropenia in different studies are presented in annex tables 2 and 3. Included studies used heterogeneous criteria to define risk levels in their samples (Table 1).
At present, there are three available scales for estimating the risk of medical complications in patients with febrile neutropenia: the rules developed by Talcott et al. [3] and the MASCC [13] (from 0 to 26 points, a score ≥ 21 is predictive of low risk) and CISNE [14] (from 0 to 8 points, with three levels of risk: I or low ≥ 2; II or intermediate ≥ 4; and III or high > 4). These validated scoring systems assume neutropenia and fever status for certain patients without regard to the severity or duration of the neutropenia as predictors of medical complications, which could require or prolong hospitalization.
Validated criteria have been used to identify patients with febrile neutropenia at low risk in clinical trials (since 2008) and observational studies (since 2010). Except for the studies by Talcott et al. [3], Mizuno et al. [15], and Rolston et al. [16], which use the Talcott criteria [3], all other clinical trials and observational studies have used the MASCC score, generally with a cutoff of 21 points to define low risk (Kern et al. [1] used a cutoff of 20 points). No included studies used the CISNE scale. However, some studies have employed clinical, biochemical, and sometimes psychosocial criteria to establish risk levels, including the studies by Rubenstein et al. [10] and Hidalgo et al. [6]. Likewise, Weycker et al. [21] also did not use a specific scale in their retrospective cohort study, instead choosing treatment arms based on the diagnostic setting.
In all included clinical trials, the ratio of patients with hematological neoplasms (leukemia or lymphoma) to solid tumors was approximately 1:4 to 1:3. All but two observational studies assessing a low-risk intervention protocol included only patients with solid tumors: the ratio in the study by Poprawski et al. [20] was 1:3, while the distribution between tumor types in the study by Goodman et al. [22] was roughly equal.
Antibiotic treatments used, administration routes, and treatment settings are presented in annex tables 2 and 3. Moreover, the samples of almost all clinical trials and observational studies included 25 to 38% of people being treated with colony-stimulating factors (CSFs), except for Innes et al. [5], who excluded them.
Between 6 and 20% of patients treated on an ambulatory basis were readmitted.
Analysis of efficacy
As for the results of the efficacy analysis, Rubenstein et al. [9], Minotti et al. [6], and Sebban et al. [4] did not find differences between oral versus intravenous administration in low-risk patients with neutropenia. The latter two studies evaluated a monotherapy antibiotic regimen. Malik et al. [9] found that oral fluoroquinolone monotherapy was equally effective whether administered in hospitals or other settings. Rapoport et al. [8] likewise obtained similar results in patients receiving classical inpatient care or early discharge with ambulatory therapeutic management. In line with these results, Hidalgo et al. [6] did not find differences in treatment response between an inpatient i.v. double regimen versus oral monotherapy, administered in ambulatory care following good response to the first dose in hospital and early discharge. These findings were also reproduced by Innes et al. in 2003 [5], who compared double i.v. therapy in-hospital versus double oral treatment in ambulatory care. For their part, Talcott et al. [3] did not observe a higher rate of complications using an ambulatory strategy. Finally, Kern et al. [2] reported similar results between double versus monotherapy in the ambulatory setting.
In the observational studies, Mizuno et al. [15] suggested that telephone follow-up could be an effective strategy to support ambulatory management. Cooksley et al. [23] drew similar conclusions, speculating that ambulatory treatment could be more feasible if clinical oncological support were available; this group provided such support in the form of an oncologist on call through a 24 h hotline. Rolston et al. [16] evaluated an early discharge strategy with gatifloxacin monotherapy 400 mg versus moxifloxacin 400 mg, suggesting that quinolone monotherapy could be a safe and effective intervention in low-risk patients selected using the Talcott criterio [3] and the MASCC score, respectively. In addition, Hocking et al. [19] implemented an ambulatory management strategy aligned with Australian practice guidelines in 2012, concluding that this could be a valid strategy for selected patients with solid tumors and low-risk febrile neutropenia. In their retrospective analysis, Weycker et al. [21] obtained similar results in a sample of patients with mostly solid tumors, a small proportion of whom were treated exclusively as outpatients. Goodman et al. in 2017 [22] and Poprawski in 2018 [20] also reached the same conclusion based on their studies, with ratios of 1:1 and 1:3, respectively, of hematological to solid tumors. Goodman et al. [22] noted that applying social and non-medical criteria in addition to the MASCC score complicated ambulatory treatment approaches in low-risk patients with febrile neutropenia. In the same line as Hocking et al. [19], Lingaratnam et al. [17] analyzed the successful implementation of the Australian protocol in a center in Victoria. Even though there was a sizable proportion of patients who could not initially be classified as low risk, their evolution permitted a change to the oral treatment group, and none presented treatment failure.
Cost-effectiveness analysis
Our search strategy yielded a total of 8 articles with a focus on cost-effectiveness: 1 clinical trial, 2 cohort studies, and 5 other observational studies. The characteristics of the studies (research group, year of publication, country, definition of febrile neutropenia, mean age, design, perspective, source of economic data, length of hospital stay, direct and indirect costs, and summary of results) are shown in annex table 4.
This analysis did not use data from other included studies that mentioned cost savings without providing a detailed explanation of the methods used to calculate them, as the 8 studies that are included in this analysis did.
Three studies analyzed the direct costs derived from inpatient management of febrile neutropenia. Kuderer et al. [24] reported that the health care expenditure from each episode of febrile neutropenia arose mainly from the costs of the hospital stay along with associated infections and comorbidities. Mayordomo et al. [25] went into more detail, itemizing the costs for each classical intervention and showing that hospitalization accounted for 79% of the costs, followed by antibiotic treatment (10%), CSF (5%), complementary tests (4%), and transfusions (1%). Larger costs were incurred (in-hospital stay, interventions, and complementary tests) in patients with lymphoma compared to breast or lung cancers. Finally, O’Brien et al. [26] added that health care costs in patients aged 65 years or older were significantly higher.
Five included studies assessed the economic impact of an ambulatory-based treatment strategy. In 2000, Elting et al. [27] found that an ambulatory strategy incurred lower costs than i.v. treatment during admission. In their 2008 cohort [28], the same group found no differences in the efficacy of hospital versus ambulatory treatment, and even though just 21% of the ambulatory group were readmitted, the cost in that arm was double. In 2011, Hendricks et al. [29] reported savings of USD 5354 using an ambulatory strategy with early discharge, considering the costs derived from the initial emergency department consultation, complementary studies, home interventions, pharmacological treatment, and daily nurse home visits. In 2017, Teh et al. [30] reported 2.65 times lower costs after applying an ambulatory strategy in low-risk patients compared to a comparable historic cohort in the same hospital. Finally, Borget et al. [31] aimed to identify where the largest economic burden came from, comparing the standard hospital strategy versus outpatient treatment in hospital and ambulatory treatment outside of it, reporting successive decreases in costs in each setting.
Analysis of patient-reported quality of life
Patients’ perceived health was only specifically analyzed in relation to ambulatory treatment strategies by Teuffel et al. in 2011 [32], who asked cancer patients to hypothetically consider what their preferred treatment strategy would be if they presented febrile neutropenia during the course of their disease. Authors observed that patients generally preferred early discharge plus ambulatory treatment with oral rather than i.v. administration. Patients were willing to renounce up to 10 weeks of their life and pay between USD 255 and USD 327 more if they could avoid hospitalization. However, in individual interviews, some perceptions changed substantially. Details of this research are presented in annex table 5.
In addition, two of the included clinical trials—one by the Talcott group in 2011 and another by Sebban et al. in 2008 [4]—performed a simple quality of life analysis using two questionnaires. The first applied the European Organization for Research and Treatment of Cancer quality of life questionnaire (EORTC QLQ-C30), a validated, self-administered instrument, finding a significant reduction in the perception of pain in patients from the ambulatory treatment arm. The second applied a quality of life questionnaire 24 h after finalizing treatment, finding no differences between intervention groups.
Discussion
Since at least the early 1990s, with Rubenstein et al. [10], and possibly even before then, there has been recognition that the risk of complications is heterogeneous in populations with febrile neutropenia. The few studies available on this topic have been characterized by the diversity of their aims, approaches, and patient selection criteria. Factors contributing to this heterogeneity include evolving knowledge on the entity; the emergence of new oncological treatments (chemotherapies, CSFs, antibiotics); the growing tendency to simplify treatments to favor adherence and tolerability and to reduce adverse effects; and the decentralization of health care away from hospitals due to associated comorbidity, impaired quality of life, and increased cost.
Indeed, even the definition of fever itself and the best place to measure it have changed several times since Carl Wunderlich’s early work on it in 1868. This branching evolution is evident in the current definitions of the Infectious Diseases Society of America and other societies in South America, Europe, and Asia, and in the different ways that our included studies identify it; however, it is not necessarily reflected in the publication year of our included studies.
These changes are also reflected in the fact that Minotti et al. [7] excluded patients with neutrophil counts under 300/μL because at that time, severe neutropenia, especially with neutrophil counts of less than 100/μL, was considered a marker of high risk. Later studies do not reflect the application of this criterion. Indeed, the interpretation of our results must consider the variations in the neutrophil count of included and excluded patients. Kern et al. [1], Sebban et al. [4], Innes et al. [5], Hidalgo et al. [6], and Malik et al. [9] all included patients with moderate neutropenia (500–1000/μL) with the potential to become severe neutropenia (< 500/μL). However, only Malik et al. [9] clarified that the patients whose neutrophil counts did not drop below the 500/μL threshold were excluded. However, given the inclusion of patients with critical levels of neutropenia in other studies, this distinction may be irrelevant.
Another fact suggestive of the diversity of selection criteria is that despite criticism of Talcott’s group and the MASCC and CISNE scales for not considering the neutrophil count as a criterion for risk assessment, the risk associated with this aspect does not seem to determine the appropriateness of managing neutropenia on an ambulatory basis. Indeed, good outcomes were achieved with simplified therapies in the ambulatory setting regardless of the criteria for selecting candidates. In the studies that did report the proportion of patients with neutrophil counts that dipped below 100/μL (Kern et al. [2], Talcott et al. [3], Hidalgo et al. [6], Minotti et al. [7], Malik et al. [9], and Rubenstein et al. [10]), these proportions were described as similar between groups in all studies but Rubenstein et al.’s, who did not specify.
Regarding the risk assessment scales, the ASCO 2018 [11] guidelines recommend the use of MASCC followed by CISNE, while the SEOM 2018 [12] guidelines prefer only the CISNE. However, the included studies did not evaluate the latter scale as a method to identify candidates for ambulatory care. Moreover, CISNE has not been validated in hematological patients, restricting the subgroups eligible for less aggressive interventions. Furthermore, Ahn et al. [33] demonstrated that the MASCC had greater discriminatory power than the CISNE for detecting low-risk patients, with greater sensitivity and negative predictive value, resulting in a smaller likelihood of including patients at true high risk of complications in ambulatory regimes. Thus, in 2013, Ahn et al. [34] already proposed procalcitonin values as an additional parameter for the MASCC to refine the probability of presenting bacteremia or septic shock, in patients at both low and high risk. Even within the latter group, the authors recognized heterogeneous prognoses, calling into question the need for indiscriminately applying aggressive management.
The evidence on antibiotic treatment approaches, both for double therapy with amoxycillin/clavulanic acid (or clindamycin in patients with allergies) plus quinolone and for monotherapy with fluoroquinolone, shows good efficacy outcomes in and outside of the hospital setting (Kern et al. [2], Sebban et al. [4], Hidalgo et al. [6], Minotti et al. [7], Malik et al. [9]). Despite these results, the ASCO guidelines [11] continue advising against fluoroquinolone monotherapy for ambulatory management of low-risk patients, while SEOM affirms that the only alternative in penicillin-allergic patients is hospital admission and i.v. treatment.
In 20 to 30% of febrile neutropenia cases, the germs responsible are identified, predominantly gram-positive rather than gram-negative bacteria, followed by polymicrobial and fungal infections (Malik et al. [9], Rapoport et al. [8], Hidalgo et al. [6], Minotti et al. [7], Sebban et al. [4]). These results are consistent with other available studies in the literature.
With respect to the use of CSFs, it has been known for decades, as reported by studies like Mather et al. in 1994 [35], that these interventions are capable of reducing the mean duration of the neutropenia by 1 day as well as the total time needed for resolution, although not the total days of fever. No study has explained how authors decided to administer this treatment, describing the criteria, type of neoplasm, level of neutrophils, or timing of administration (from the last session of chemotherapy or emergency presentation). Similarly, the literature has not explored how CSFs may have influenced the outcome (patient recovery or the success of the treatment strategy). There are also gaps in research investigating whether prescribing CSFs would result in savings given the implicit pharmacological costs associated with hospitalizing these patients in a hospital or their home, and whether it would be possible to reduce the duration of antibiotic therapy in patients with a fever of unknown origin and negative culture.
Several strategies for ambulatory follow-up have been evaluated, including home visits from health professionals, hotlines, scheduled telephone calls, and outpatient hospital visits. However, no studies have compared these interventions with each other to assess treatment response, adverse effects, cost-efficiency, or quality of life. These aspects could be the focus of future lines of research.
Although teenagers were not included as population in our systematic review, because we excluded studies with population under 19 years old in our criteria research, in many countries, they are treated around 16 years old in emergency departments as adults. Authors, such as Klastersky et al. [36], carried on an early discharge strategy after 24 h, til 48 h in many of the episodes of febrile neutropenia in people that includeed 16 years old teenagers [36].
Their results show that we could safely proceed in low-risk teenagers according to MASK score, in the same way as adults with an oral combination therapy of peniciline and quinolones, if not allergic, with a proposed followed up by phone, temperature control each/6 h, and blood control test each 48 h until accomplishing criteria of recovering. We should highlight in this investigation team, they first give a definition more than absence of hospital readmission as a criteria of succes of oral empirical therapy as 5 days without fever, no clinic symptoms, and signs of infection and pathogen erradication.
Within the sphere of ambulatory management, four studies proposed that patients receive hemograms every 2 to 3 days after discharge, at least until achieving neutrophil counts of 1000/μL, indicating mild neutropenia (Goodman et al. [22], Kern et al. [2], Sebban et al. [4], Hocking et al. [19]). Although several studies have evaluated the cost of ambulatory follow-up, there has been no calculation of the specific cost of such controls, which could generate substantial amounts of research information. It may be appropriate to restrict these control visits to cases with poor evolution or to limit them to one visit at the end of treatment.
Another strategy that could be analyzed for its efficacy and cost-efficiency would be the performance of a preliminary assessment of oncological patients’ household situation (including family support) by home hospital team, prior to initiating chemotherapy. This assessment could help facilitate decision-making in emergency departments around managing these patients.
Many studies have limited ambulatory approaches to patients who live at some distance from the reference center or hospital (Kern et al. [2], Hidalgo et al. [6], Talcott et al. [3], Minotti et al. [7], Rubenstein et al. [10], Rolston et al. [18], Lingaratnam et al. [17]). To reduce the population of low-risk patients excluded from this strategy, future research could also assess the efficacy, patient satisfaction, and cost-efficiency associated with strategies in coordination with primary health care teams, for patients who live far from the hospital and whose only limitation for inclusion in an ambulatory regime is distance.
Available studies suggest than an ambulatory strategy for low-risk patients with febrile neutropenia is cost-efficient as well as effective. This is true for interventions assessed both in clinical trials (Hendricks et al. [29], Elting et al. [27]) and in prospective studies, for example, in those evaluating the implementation of new protocols compared to data from historical cohorts fulfilling the same criteria (Teh et al. [30], Mayordomo et al. [25]). Economic studies should be considered in light of potential differences in the source of economic data and their perspective. Establishing the most appropriate design to allow comparison with other studies can be complicated because health systems are not managed the same across different countries, nor is access to different therapeutic options the same in privately managed systems. Thus, in future studies, it will be necessary to establish quality criteria in order to favor reliability and comparability of the economic data obtained.
With regard to quality of life, Teuffel et al. [37] suggested that patients prefer ambulatory strategies, although data did not show differences in the short quality of life evaluations performed in two clinical trials in people with febrile neutropenia. In any case, more structured trials that specifically aim to assess this domain are needed before drawing any firm conclusions.
Despite the positive evidence, fewer patients benefit from ambulatory strategies than expected (for clinical, socioeconomic, and geographic reasons). Health services still seem reluctant to implement the action protocols in low-risk patients that are recommended by national and international clinical practice guidelines, even though these tightly restrict the application of ambulatory models.
Available evidence on efficacy, quality of life, and cost-efficiency seems to support ambulatory approaches for low-risk patients with febrile neutropenia. The main societies and services involved in managing these patients should establish more standardized priorities and criteria to guide future research and reach more definitive conclusions. Studies are needed to analyze the facillitators and barriers for the instigation and maintenance of an ambulatory febrile neutropenia management programmes.
Data availability
N/A.
Code availability
N/A.
Change history
15 January 2024
Missing Open Access funding information has been added in the Funding Note.
References
Chisholm J, Dommett R (2006) The evolution towards ambulatory and day-case management of febrile neutropenia. Br J Haematol 135(1):3–16
Kern W, Marchetti O, Drgona L, Akan H, Aoun M, Akova M, de Bock R, Paesmans M, Viscoli C, Calandra T (2013) Oral antibiotics for fever in low-risk neutropenic patients with cancer: a double-blind, randomized, multicenter trial comparing single daily moxifloxacin with twice daily ciprofloxacin plus amoxicillin/clavulanic acid combination therapy—EORTC infectious diseases group trial XV. J Clin Oncol 31(9):1149–1156
Talcott J, Yeap B, Clark J, Siegel R, Loggers E, Lu C, Godley P (2011) Safety of early discharge for low-risk patients with febrile neutropenia: a multicenter randomized controlled trial. J Clin Oncol 29(30):3977–3983
Sebban C, Dussart S, Fuhrmann C, Ghesquieres H, Rodrigues I, Geoffrois L, Devaux Y, Lancry L, Chvetzoff G, Bachelot T, Chelghoum M, Biron P (2008) Oral moxifloxacin or intravenous ceftriaxone for the treatment of low-risk neutropenic fever in cancer patients suitable for early hospital discharge. Support Care Cancer 16(9):1017–1023. https://doi.org/10.1007/s00520-007-0383-z
Innes HE, Smith DB, O’Reilly SM, Clark PI, Kelly V, Marshall E (2003) Oral antibiotics with early hospital discharge compared with in-patient intravenous antibiotics for low-risk febrile neutropenia in patients with cancer: a prospective randomised controlled single centre study. Br J Cancer 89(1):43–49. https://doi.org/10.1038/sj.bjc.6600993
Hidalgo M, Hornedo J, Lumbreras C, Trigo JM, Colomer R, Perea S, Gómez C, Ruiz A, García-Carbonero R, Cortés-Funes H (1999) Outpatient therapy with oral ofloxacin for patients with low risk neutropenia and fever: a prospective, randomized clinical trial. Cancer. 85(1):213–219
Minotti V, Gentile G, Bucaneve G, Iori AP, Micozzi A, Cavicchi F, Barbabietola G, Landonio G, Menichetti F, Martino P, Del Favero A (1999) Domiciliary treatment of febrile episodes in cancer patients: a prospective randomized trial comparing oral versus parenteral empirical antibiotic treatment. Support Care Cancer 7(3):134–139. https://doi.org/10.1007/s005200050243
Rapoport BL, Sussmann O, Herrera MV, Schlaeffer F, Otero JC, Pavlovsky S, Iglesias L, Stein G, Charnas R, Heitlinger E, Handschin J (1999) Ceftriaxone plus once daily aminoglycoside with filgrastim for treatment of febrile neutropenia: early hospital discharge vs. standard in-patient care. Chemotherapy. 45(6):466–476. https://doi.org/10.1159/000007240
Malik IA, Khan WA, Karim M, Aziz Z, Khan MA (1995) Feasibility of outpatient management of fever in cancer patients with low-risk neutropenia: results of a prospective randomized trial. Am J Med 98(3):224–231. https://doi.org/10.1016/s0002-9343(99)80367-2
Rubenstein EB, Rolston K, Benjamin RS, Loewy J, Escalante C, Manzullo E, Hughes P, Moreland B, Fender A, Kennedy K et al (1993) Outpatient treatment of febrile episodes in low-risk neutropenic patients with cancer. Cancer. 71(11):3640–3646. https://doi.org/10.1002/1097-0142(19930601)71:11<3640::aid-cncr2820711128>3.0.co;2-h
Taplitz R, Kennedy E, Bow E, Crews J, Gleason C, Hawley D, Langston A, Nastoupil L, Rajotte M, Rolston K, Strasfeld L, Flowers C (2018) Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical Practice Guideline Update. J Clin Oncol 36(14):1443–1453
Carmona-Bayonas A, Jimenez-Fonseca P, de Castro E, Mata E, Biosca M, Custodio A, Espinosa J, Vázquez E, Henao F, Ayala de la Peña F (2019) SEOM clinical practice guideline: management and prevention of febrile neutropenia in adults with solid tumors (2018). Clin Transl Oncol 21(1):75–86
Klastersky J, Paesmans M, Rubenstein EB, Boyer M, Elting L, Feld R, Gallagher J, Herrstedt J, Rapoport B, Rolston K, Talcott J (2000) The Multinational Association for Supportive Care in Cancer risk index: a multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol 18(16):3038–3051. https://doi.org/10.1200/JCO.2000.18.16.3038
Carmona-Bayonas A, Jiménez-Fonseca P, Virizuela Echaburu J, Antonio M, Font C, Biosca M et al (2015) Prediction of serious complications in patients with seemingly stable febrile neutropenia: validation of the Clinical Index of Stable Febrile Neutropenia in a prospective cohort of patients from the FINITE study. J Clin Oncol 33(5):465–471
Mizuno T, Katsumata N, Mukai H, Shimizu C, Ando M, Watanabe T (2007) The outpatient management of low-risk febrile patients with neutropenia: risk assessment over the telephone. Support Care Cancer 15(3):287–291 Epub 2006 Aug 29
Rolston K, Manzullo E, Elting L, Frisbee-Hume S, McMahon L, Theriault R, Patel S, Benjamin R (2006) Once daily, oral, outpatient quinolone monotherapy for low-risk cancer patients with fever and neutropenia: a pilot study of 40 patients based on validated risk-prediction rules. Cancer. 106(11):2489–2494
Lingaratnam S, Mellerick A, Worth L, Green M, Guy S, Kirsa S, Slavin M, Renwick W, Filshie R, Thursky K (2013) Feasibility of early discharge strategies for neutropenic fever: outcomes of a Victorian organisational readiness assessment and pilot. Intern Med J 43(9):979–986
Rolston K, Frisbee-Hume S, Patel S, Manzullo E, Benjamin R (2010) Oral moxifloxacin for outpatient treatment of low-risk, febrile neutropenic patients. Support Care Cancer 18(1):89–94
Hocking C, Taylor A, Hayward A (2013) Early discharge and ambulatory care of low-risk patients with neutropenic fever in Australia. Intern Med J 43(5):591–595
Poprawski D, Chan J, Barnes A, Koczwara B (2018) Retrospective audit of neutropenic fever after chemotherapy: how many patients could benefit from oral antibiotic management? Intern Med J 48(12):1533–1535
Weycker D, Barron R, Kartashov A, Legg J, Lyman G (2014) Incidence, treatment, and consequences of chemotherapy-induced febrile neutropenia in the inpatient and outpatient settings. J Oncol Pharm Pract 20(3):190–198
Goodman L, Estfan B, Montero A, Kunapareddy G, Lau J, Gallagher E, Best C, Tripp B, Moeller M, Bolwell B, Stevenson J (2017) Improving the management of patients with low-risk neutropenic fever at the Cleveland Clinic Taussig Cancer Institute. J Oncol Pract 13(3):e259–e265
Cooksley T, Campbell G, Al-Sayed T, La Mola L, Berman R (2018) A novel approach to improving ambulatory outpatient management of low risk febrile neutropenia: an Enhanced Supportive Care (ESC) clinic. Support Care Cancer 26(9):2937–2940
Kuderer N, Dale D, Crawford J, Cosler L, Lyman G (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 106(10):2258–2266
Mayordomo J, López A, Viñolas N, Castellanos J, Pernas S, Domingo Alonso J, Frau A, Layola M, Antonio Gasquet J, Sánchez J; ENIA Study Group (2009) Retrospective cost analysis of management of febrile neutropenia in cancer patients in Spain. Curr Med Res Opin 25(10):2533–2542
O’Brien C, Fogarty E, Walsh C, Dempsey O, Barry M, Kennedy M, McCullagh L (2015) The cost of the inpatient management of febrile neutropenia in cancer patients—a micro-costing study in the Irish healthcare setting. Eur J Cancer Care (Engl) 24(1):125–132
Elting L, Rubenstein E, Rolston K, Cantor S, Martin C, Kurtin D, Rodriguez S, Lam T, Kanesan K, Bodey G (2000) Time to clinical response: an outcome of antibiotic therapy of febrile neutropenia with implications for quality and cost of care. J Clin Oncol 18(21):3699–3706
Elting L, Lu C, Escalante C, Giordano S, Trent J, Cooksley C, Avritscher E, Shih Y, Ensor J, Bekele B, Gralla R, Talcott J, Rolston K (2008) Outcomes and cost of outpatient or inpatient management of 712 patients with febrile neutropenia. J Clin Oncol 26(4):606–611
Hendricks A, Loggers E, Talcott J (2011) Costs of home versus inpatient treatment for fever and neutropenia: analysis of a multicenter randomized trial. J Clin Oncol 29(30):3984–3989
Teh B, Brown C, Joyce T, Worth L, Slavin M, Thursky K (2018) Safety and cost benefit of an ambulatory program for patients with low-risk neutropenic fever at an Australian centre. Support Care Cancer 26(3):997–1003
Borget I, Antoun S, Chachaty E, Gachot B, Alibay A, Di Palma M, Merad M (2014) Modalities of management of cancer patients with febrile neutropenia in the oncology emergency unit of Gustave-Roussy and their related costs. Bull Cancer 101(10):925–931
Teuffel O, Cheng S, Ethier M, Diorio C, Martino J, Mayo C, Wing R, Sung L, Alibhai S (2012) Health-related with different management strategies for febrile neutropenia in adult cancer patients. Support Care Cancer 20(11):2755–2764
Ahn S, Rice T, Yeung S, Cooksley T (2018) Comparison of the MASCC and CISNE scores for identifying low-risk neutropenic fever patients: analysis of data from three emergency departments of cancer centers in three continents. Support Care Cancer 26(5):1465–1470
Ahn S, Lee Y, Lim K, Lee J (2013) Adding procalcitonin to the MASCC risk-index score could improve risk stratification of patients with febrile neutropenia. Support Care Cancer 21(8):2303–2308
Mather D, Lieschke G, Green M (1994) Filgastrim in patients with chemotherapy-induced febrile neutropenia. Ann Intern Med 121:492–501
Klastersky J, Paesmans M, Georgala A, Muanza F, Plehiers B, Dubreucq L et al (2006) Outpatient oral antibiotics for febrile neutropenic cancer patients using a score predictive for complications. J Clin Oncol 24(25):4129–4134. Disponible en:. https://doi.org/10.1200/JCO.2005.03.9909
Teuffel O, Ethier M, Alibhai S, Beyene J, Sung L (2011) Cost effectiveness of outpatient treatment for febrile neutropaenia in adult cancer patients. Br J Cancer 104(9):1377–1383
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by EF-Q. The first draft of the manuscript was written by EF-Q, DO-B, and CL-Q. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
EF-Q had the idea for the article; EF-Q, DO-B, and CL-Q carried out the bibliographic search and data analysis; and EF-Q, DO-B, and CL-Q wrote and/or critically reviewed the article.
Corresponding author
Ethics declarations
Ethics approval
Approval was obtained from the ethics committee of University Miguel Hernandez de Elche. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate
Personal information is unidentifiable in this present study; and patient consent cannot be obtained.
Consent for publication
N/A.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 344 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Forcano-Queralt, E., Lemes-Quintana, C. & Orozco-Beltrán, D. Ambulatory management of low-risk febrile neutropenia in adult oncological patients. Systematic review. Support Care Cancer 31, 665 (2023). https://doi.org/10.1007/s00520-023-08065-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-08065-y