Abstract
Purpose
Following curative-intent therapy of lung cancer, many survivors experience dyspnea and physical inactivity. We investigated the feasibility, acceptability, safety, and potential efficacy of inspiratory muscle training (IMT) and walking promotion to disrupt a postulated “dyspnea-inactivity” spiral.
Methods
Between January and December 2022, we recruited lung cancer survivors from Kaiser Permanente Colorado who completed curative-intent therapy within 1–6 months into a phase-IIb, parallel-group, pilot randomized trial (1:1 allocation). The 12-week intervention, delivered via telemedicine, consisted of exercise training (IMT + walking), education, and behavior change support. Control participants received educational materials on general exercise. We determined feasibility a priori: enrollment of ≥ 20% eligible patients, ≥ 75% retention, study measure completion, and adherence. We assessed acceptability using the Telemedicine-Satisfaction-and-Usefulness-Questionnaire and safety events that included emergency department visits or hospitalizations. Patient-centered outcome measures (PCOMs) included dyspnea (University-of-California-San-Diego-Shortness-of-Breath-Questionnaire), physical activity (activPAL™ steps/day), functional exercise capacity (mobile-based-six-minute-walk-test), and health-related quality of life (HRQL, St.-George’s-Respiratory-Questionnaire). We used linear mixed-effects models to assess potential efficacy.
Results
We screened 751 patients, identified 124 eligible, and consented 31 (25%) participants. Among 28 participants randomized (14/group), 22 (11/group) completed the study (79% retention). Intervention participants returned > 90% of self-reported activity logs, completed > 90% of PCOMs, and attended > 90% of tele-visits; 75% of participants performed IMT at the recommended dose. Participants had high satisfaction with tele-visits and found the intervention useful. There was no statistically significant difference in safety events between groups. Compared to control participants from baseline to follow-up, intervention participants had statistically significant and clinically meaningful improved HRQL (SGRQ total, symptom, and impact scores) (standardized effect size: -1.03 to -1.30).
Conclusions
Among lung cancer survivors following curative-intent therapy, telemedicine-based IMT + walking was feasible, acceptable, safe, and had potential to disrupt the “dyspnea-inactivity” spiral. Future efficacy/effectiveness trials are warranted and should incorporate IMT and walking promotion to improve HRQL.
Trial Registration: ClinicalTrials.gov NCT05059132.
Similar content being viewed by others
Introduction
Dyspnea is an important patient-centered outcome impacting health-related quality of life (HRQL) in lung cancer [1]. High dyspnea burden may reduce functional exercise capacity [2] and survival [3]. Compared to pretreatment, dyspnea worsens among lung cancer survivors following curative intent therapy [4,5,6,7] and can persist for years posttreatment, regardless of treatment modality [8,9,10]. Pathophysiologically, dyspnea can occur due to resected or damaged lung tissue, lost or damaged nerve fibers [11], and activation or increased stimulation of peripheral sensors [12]. Along with alterations in the chest wall, respiratory muscle, and airway [13], these changes can culminate in neuromechanical dissociation and increase central ‘corollary discharge’ [13]. In fact, clinically significant dyspnea exists among up to 70–80% of lung cancers survivors within six months following curative intent therapy [5, 14] and 60% among those ≥ 1 year/s posttreatment [15]. As such, dyspnea is a modifiable factor that could be targeted to improve HRQL following curative intent therapy [16].
A psychological consequence of dyspnea is fear, panic, and anxiety, particularly with exertion [17]. A behavioral consequence of dyspnea [17] is avoidance of physical activity and exercise [18], with physical inactivity associated with poor HRQL [19] and worse survival in early stage lung cancer [20]. The American College of Chest Physicians identified a need for strategies to improve HRQL following curative intent therapy of lung cancer [21]. Accordingly, we proposed a conceptual model of a vicious cycle of “dyspnea-inactivity” downward health spiral that needs to be promptly disrupted for this unique [2] and growing population of cancer survivors [22].
Inspiratory muscle training (IMT) is a resistance-based exercise training regimen to improve strength and endurance of respiratory muscles. IMT alleviates dyspnea for patients with chronic obstructive pulmonary disease (COPD) [23], can be performed in patients’ homes, and is a promising strategy to meet lung cancer survivors’ needs for remotely-delivered rehabilitation [24]. In addition, walking is the preferred physical activity modality among lung cancer survivors [25]. Therefore, IMT and walking promotion may disrupt the vicious cycle of”dyspnea-inactivity.” In this project, we conducted a pilot randomized trial of a telemedicine-based rehabilitation strategy consisting of IMT + walking with lung cancer survivors following curative intent therapy. We hypothesized that IMT + walking is feasible, acceptable, safe, and compared to education only, could improve dyspnea control, physical activity, functional exercise capacity, and HRQL.
Methods
Trial design & study overview
We registered this protocol (NCT05059132) and designated physical activity as a primary outcome. We applied the ORBIT model for developing behavioral treatments [26] and conducted a phase IIb, parallel group, pilot randomized trial (1:1 allocation). This study received approval and waiver of signed informed consent from the Kaiser Permanente Colorado Institutional Review Board (#1,717,517–12). Participants’ verbal informed consent was obtained by telephone following a discussion of the study, contained all the required elements of informed consent, and documented in REDCap – a secure electronic data management system [27]; a copy of the informed consent form was sent to participants thereafter. To report findings, we followed the Consolidated Standards of Reporting Trials, pilot extension [28].
Recruitment & participants
Between January and December 2022, we recruited patients from Kaiser Permanente Colorado (KPCO), an integrated healthcare system that provides health insurance and clinical services to > 500,000 individuals in the metropolitan Denver and surrounding Colorado communities. We used a multi-modal recruitment approach: 1) identification of patients with receipt of curative intent therapy; 2) new referrals to the pulmonology, surgery, or radiation oncology departments for newly-diagnosed or suspected lung cancer; and 3) patients presented at a weekly lung cancer/nodule conference. To facilitate recruitment, we developed an algorithm of codes and local chemoradiation protocols, incorporating relevant time periods and exclusionary conditions (Online Resource 1). We reviewed the records of patients identified and sent recruitment letters to those deemed potentially eligible. We allowed two weeks for patients to decline recruitment and reached out to those who did not decline.
We included adult stage I-IIIA lung cancer survivors who completed the primary mode of curative intent therapy (i.e., surgical resection, definitive radiation, or concurrent chemoradiation) in the prior 1–6 months. We excluded patients with: 1) recent major cardiovascular events or acute asthma exacerbation; 2) spontaneous pneumothorax within 12 months; 3) neurologic or movement disorders; 4) dementia; 5) estimated < 6-months life expectancy or in hospice care; 6) no internet access; 7) inactive KPCO membership; 8) any preferred language other than English; or 9) unwilling to wear activity trackers. We included patients not willing to participate in telemedicine, as we would allow in-person visits, if needed. We obtained demographic, physiologic, and clinical characteristics from the electronic medical records.
Randomization
Participants who completed baseline patient-centered outcome measures (PCOMs) were randomized in permuted blocks of four, stratified by receipt of surgical or non-surgical treatment, and allocated 1:1 to the IMT + walking (intervention) or education only (control) groups. A computer-generated allocation sequence was uploaded onto REDCap [27]. The study investigators, but not participants, interventionist, or outcome assessor, were blinded to group allocation.
IMT + walking (Intervention)
Participants in the IMT + walking group received an intervention designed with essential components of pulmonary rehabilitation (i.e., exercise training, education, behavioral support) [29], delivered in six tele-visits over 12 weeks. Exercise training consisted of adapted IMT [30] + walking [31], guided by exercise recommendations to improve HRQL for cancer survivors [i.e., moderate-vigorous intensity physical activity (MVPA) ≥ 60–90 min/week for 12 weeks] [32]. Education focused on the potential of IMT + walking to improve dyspnea control, function, HRQL, and incorporated patient educational materials on dyspnea [33], IMT, and physical activity [34]. Behavioral support was informed by the 2018 Physical Activity Guidelines Advisory Committee (PAGAC) Scientific Report from the US Department of Health and Human Services, which concluded that “strong evidence demonstrates that behavior change theory and techniques are effective for increasing physical activity levels in general adult populations” [35]. We adapted Bandura’s Social Cognitive Theory (SCT) [36], a framework identified to be effective in this Scientific Report [35] with supporting evidence among cancer survivors [37]. The SCT postulates that knowledge of health risks and benefits initiates the process of possible behavior change, with behavior influenced by 1) perceived self-efficacy, 2) facilitators and impediments, 3) outcome expectations, and 4) goals. Guided by the SCT, behavioral support incorporated behavior change techniques shown to be effective in promoting habitual exercise among general adults [35] and cancer survivors [38] – i.e., providing information on the expected benefits of IMT + walking, clear instructions on how to perform IMT to promote self-efficacy, setting achievable activity goals, graded tasks, self-monitoring, identifying barriers and facilitators, problem-solving, and feedback. We provided descriptions of how the SCT and behavior change techniques were applied in the intervention in Table 1 and Online Resource 2a-b. We did not combine behavior change theories and avoided behavior change techniques identified by the PAGAC to be likely ineffective (e.g., general encouragement) [35].
Tele-visits were scheduled at weeks 1, 3, 5, 7, 9, and 12 following randomization and lasted 30–60 min/visit. All six visits involved live interventionist interaction via telemedicine, with IMT and Fitbit devices sent via parcel services. The initial tele-visit focused on introduction to IMT + walking and expected benefits, instructions on how to perform IMT, setting activity goals for the coming weeks, and the use of activity logs and activity trackers for self-monitoring. The subsequent tele-visits focused on behavioral support, with collaborative review of activity goals achieved, identification of barriers and facilitators, problem-solving, and feedback.
Inspiratory muscle training
IMT was performed using Threshold IMT™ devices (Philips Healthcare), with instructions provided via video-visits to allow observation, feedback, and ensure IMT proficiency. Participants were instructed to adjust IMT resistance to a perceived rating of exertion of “somewhat-hard to hard” (4–6 on the 0–10 modified Borg scale), perform unsupervised IMT 10–15 min twice/day (or ≥ 20 min/daily) for ≥ 5 days/week (i.e., IMT ≥ 100 min/week), with progression to higher resistance as tolerated. Once participants have demonstrated proficiency with IMT in video-visit/s, telephone visits were allowed if participants encountered significant technical difficulties (e.g., loss of internet signal).
Walking
Walking promotion was facilitated by patient-facing activity trackers (Fitbit Inspire 2™) for activity goal-setting, self-monitoring, and feedback. To obtain baseline step count, we sent Fitbits to participants, assisted them with device/account set-up, and instructed one-week wear prior to the initial tele-visit. The activity goal was 5–10% increases from baseline and previous weeks’ step counts. To achieve activity goals, participants were encouraged to go on ≥ 4 walks/week or increase walk durations by 5–10 min. To facilitate self-monitoring and adherence, participants were asked to access Fitbit data and self-record step counts and IMT sessions on activity logs, completed weekly, returned, and discussed at each tele-visit.
Interventionist
To deliver the intervention, a research specialist completed coursework in motivational interviewing [39] and received training in IMT with support from respiratory therapists. Microsoft Teams™ was used for video-conferencing, with telephone used as needed if participants had demonstrated adequate IMT proficiency. To ensure fidelity, the interventionist used a checklist of components (Online Resource 2a-b), with 10% of completed checklists reviewed to identify and address any challenges to delivery.
Education only (Control)
Control group participants received written educational materials – on physical activity in lung cancer [34]; sitting less/moving more [40]; and being physically active [41] – sent via email or post at weeks 1, 4, and 8. There were no additional monitoring or contact with study personnel, except at weeks 6 and 12 for study outcome assessments. These participants received IMT and Fitbit devices at study end.
Outcomes
Feasibility, Acceptability, and Safety
We determined feasibility a priori, guided by frameworks for pilot randomized rehabilitation trials [42, 43]: enrollment (i.e., ≥ 20% of eligible patients), randomization (i.e., participant willingness to be randomized), participant adherence (i.e., attendance of ≥ 75% of tele-visits; performance of ≥ 75% unsupervised IMT + walking), interventionist fidelity, measurement processes, and retention (i.e., ≥ 75%). We chose a higher-than-recommended completion threshold (70% for quality pulmonary rehabilitation) [44] due to the mostly unsupervised nature of IMT + walking, as the effects of unsupervised exercise on HRQL have been shown to be smaller compared to supervised exercise [45].
Acceptability was measured by the Telemedicine Satisfaction and Usefulness Questionnaire (1–5 Likert scale responses; scores ≥ 4 indicate acceptable satisfaction and usefulness) [46] and an exit survey on participants’ experience with the intervention. To assess safety, we identified episodes of emergency department visits or hospitalizations for participants in both groups, and any self-reported symptom/signs associated with IMT + walking.
Patient-centered outcome measures
All PCOMs were measured at time points 0 (baseline), week 6 (mid-intervention), and week 12 (end-of-intervention): dyspnea [UCSD Shortness of Breath Questionnaire (SOBQ), 0–120 point scale, higher scores indicate higher dyspnea]; anxiety [Generalized Anxiety Disorder 7-item (GAD-7), 0–21 point scale, higher scores indicate higher anxiety], sleep quality [Pittsburgh Sleep Quality Index (PSQI), 0–21 point scale, higher scores indicate worse sleep quality]; and self-reported physical activity [International Physical Activity Questionnaire – Short Form, minutes/week of walking and MVPA].
Physical activity was measured by the activPAL (4 micro, PAL™ Technologies), a valid and accurate wearable monitor to measure physical activity (stepping, step counts, step speed, postural transitions) and sedentary behavior (sitting/lying) [47] that has been used among survivors of cancer [48, 49], including lung [50]. We used a 7-day continuous wear protocol [47] and default settings/algorithms to define valid data – i.e., days with non-wear ≤ 4 h, from midnight to the next midnight, supplemented by self-reported wear/sleep logs. activPAL measures were steps/day, sedentary behavior (SB) (minutes/day), light intensity physical activity (LPA) (minutes/day with cadence < 100 steps/min), and MVPA (minutes/day with cadence ≥ 100 steps/min).
Functional exercise capacity was measured by the mobile-based six-minute walk test (m6MWT). To conduct the m6MWT, we modified the American Thoracic Society recommendations for in-person performance [51], completed the test remotely, and with live interaction (via telephone) with study personnel for monitoring. We asked participants to identify an acceptable path (flat, without traffic, and approximately 1/3rd to 1/4th the length of a typical city block – approximately 30 m) to walk back and forth. Recording of the m6MWT distance was enabled by the 6WT application (Webgearing AG). The m6MWT distance has been shown to be reliable, reproducible, and concordant (or accurate) with in-lab measures in adults [52] and patients with cardiopulmonary disease [53, 54]. We used reference equations from healthy adults [55] to calculate lower limits of normal for interpretation. Please also see Online Resource 3 for more details.
HRQL was measured by the 50-item St. George’s Respiratory Questionnaire (SGRQ), with 1) symptoms (frequency, severity); 2) activities (causing or limited by dyspnea); 3) impact (on social functioning and psychological disturbance); and total scores ranging 0–100 points; lower scores indicate improved HRQL. Online Resource 4 provides more PCOM details, including cut-off levels and minimal clinically important difference (MCID) thresholds.
Sample Size
We aimed 30–40 participants enrolled (15–20/group) based on estimates for a continuous outcome variable in a pilot trial [56] to minimize overall (pilot and efficacy) trial sample size, and detect an estimated standardized medium (0.3–0.7) effect size in an efficacy trial [56].
We used the MCID 350–1,100 steps/day (derived in COPD) [57]. We assumed a baseline activPAL 3,500 ± 2,100 steps/day [50], 40 participants randomized (20/group), 20% dropout, and with 80% power (two-sided alpha = 0.05), estimated that we would be able to detect a mean difference in response of 1,910 steps/day between groups, a very-large standardized effect size (SES 0.9) and approximately 2–5 times the MCID.
Statistical Analyses
Descriptive statistics summarized participant characteristics and two-sample t-test and Chi-square or Fisher’s exact tests compared differences between groups at baseline. Linear mixed-effects models analyzed change in PCOMs. We followed recommendations against hypothesis testing in pilot trials [28] and used unadjusted models in primary analyses, with each PCOM modeled as function of group assignment, visit week, and interaction of group by visit week. To assess potential treatment effects, changes in PCOMs from baseline were estimated and compared between groups. We used all available data. Linear mixed-effects models assumed random missing data.
To assess the validity of the “dyspnea-inactivity” spiral, we used adjusted models in secondary analyses, including participant’s age and comorbidities [58]. We did not adjust for multiple comparisons as our trial was not designed to determine efficacy/effectiveness. We used SAS/STAT analytic software (V.9.4 SAS Institute Inc).
Results
Screening and enrollment
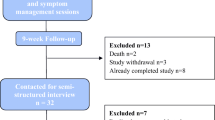
We screened 751 patients, identified 124 eligible stage I-IIIA lung cancer survivors, and consented 31 participants (25% enrollment); the most common reported reasons for non-participation were high time commitment and low interest. Three participants withdrew prior to randomization (Fig. 1). Among 28 participants randomized (14/group), the median age was 70 years; approximately 50% were women, 30% had comorbid COPD, 80% stage IA, and 60% received only surgical treatment (Table 2).
Baseline participant characteristics
Ninety-three percent of participants had abnormally-high dyspnea (SOBQ > 9 points), 93% low physical activity (activPAL < 10,000 steps/day), 86% disrupted sleep (PSQI ≥ 5 points), and 89% poor HRQL (SGRQ > 13 points). Most had minimal anxiety (GAD-7 ≤ 4 points, 64%) and adequate functional exercise capacity (m6MWT distance ≥ lower limit of normal, 83%). There were no statistically significant differences in participant characteristics, including PCOMs, between groups at baseline (Table 2).
Intervention feasibility and acceptability
Among 28 participants randomized, 22 (11/group) completed the study (79% retention); > 95% of the PCOMs were obtained, with approximately 90% of participants completing 100% of PCOMs. The most common missing PCOM was the m6MWT – with eight (12%) (four/group) not completed due to unreliable mobile phone signal, unacceptable walking space, or weather challenges.
Among 11 participants who completed the intervention, > 90% attended ≥ 75% of tele-visits, with 75% of them attending 100% of visits. Approximately 90% of activity logs were returned, with 75% of participants performing IMT at the prescribed dose – i.e., ≥ 100 min/week, and/or walking ≥ 90 min/week (Table 3a). Adherence was sustained (Online Resource 5). The research specialist delivered > 95% of the checklist items without difficulty, with no changes made during the trial. All visits were via telemedicine, mostly with video and approximately 20% with telephone due to technical challenges; there were no in-person visits.
Participants found tele-visits to be acceptable and were satisfied, noting that the software was easy to use and that tele-visits saved time. However, only 73% of participants indicated that tele-/video-visits were as satisfying as in-person visits, with 36% trusting the technology to work. Ninety to 100% of participants found instructions for IMT + walking as moderately-to-extremely helpful and would recommend the study to another or repeat a similar program. The least helpful component was written educational materials (Table 3b).
Intervention safety
Of 11 intervention participants who completed the program, six (55%) reported ≥ 1 symptom/s potentially related to IMT + walking (i.e., musculoskeletal soreness, fatigue, lightheadedness, headache, coughing, or breathlessness). There were no serious safety events (e.g., falls with walking) attributable to the intervention. The proportion of participants with emergency department visits or hospitalizations was not statistically significantly different, but appeared lower, in the intervention compared to control group (9 vs 46%, respectively, p = 0.15) (Table 3c).
Change in patient-centered outcome measures
The mean estimated changes in PCOMs from unadjusted models are in Table 4. Compared to control participants from baseline to follow up at 6- or 12-weeks, intervention participants had statistically significant (p < 0.05) improved activPAL MVPA and reduced activPAL SB at week 6 (SES 1.08 and -0.94, respectively) but not week 12, and improved HRQL (SGRQ total, symptom, and impact subdomains) at weeks 6 and 12 (SES ranged -1.03 to -1.30). The magnitude of the change differences between groups were ≥ 1–4 times the respective MCIDs and not driven by outliers (Online Resource 6). There were no statistically significant differences in the changes in SOBQ dyspnea, activPAL steps/day or LPA, anxiety, functional exercise capacity, sleep difficulties, or self-reported physical activity between groups at 6 or 12 weeks. Results were similar in adjusted compared to unadjusted models, including in the trends, directionality, and magnitude of potential treatment effects on PCOMs.
Discussion
In this pilot trial, we found that IMT + walking with behavioral support, compared to education only, was feasible, acceptable, safe, and could disrupt a “dyspnea-inactivity” spiral and improve HRQL among lung cancer survivors following curative intent therapy. These findings have important implications in efforts to reduce dyspnea and improve HRQL with this population.
The US National Academy of Medicine recommends that care for posttreatment cancer survivors include supportive services to reduce treatment adverse effects and promote health [59]. Exercise is recommended by national [60, 61] and international societies [62] for cancer survivors. However, evidence on exercise benefits is mostly derived from survivors of breast, prostate, and colorectal cancer [62], with inconsistent evidence in lung cancer [63]. A systematic review involving lung cancer survivors within 12 months of surgical treatment demonstrated benefits of aerobic and resistance training on functional exercise capacity but low-to-very-low certainty evidence on dyspnea and HRQL [64]. We propose that exercise training and rehabilitative strategies to improve HRQL for lung cancer survivors following curative intent therapy may need to consider unique characteristics, including dyspnea, high cardiopulmonary disease burden, older age, pathophysiological/biobehavioral mechanisms, and promptly target specific impairments to disrupt a downward health spiral (Fig. 2).
Conceptual Model of the Vicious Cycle of “Dyspnea-Inactivity” Downward Health Spiral Following Curative Intent Therapy of Lung Cancer. Bolded text indicates a vicious cycle of “dyspnea-inactivity.” Description [4]: Following diagnosis and curative intent therapy of lung cancer, survivors experience increased symptom burden, particularly with dyspnea due to loss of lung tissue and function, lost or damaged nerve fibers and peripheral sensors, and alterations to the neuro-respiratory system, culminating in neuromechanical dissociation and increased central ‘corollary discharge’. Consequently, many survivors avoid physical activity and exercise [17, 18], leading to a vicious cycle of “dyspnea-inactivity.” Over time, combined with worry or fear of lung cancer recurrence [73], sleep disturbance [71], fatigue [71], the adopted physical inactivity leads to deconditioning [74], impaired functional exercise capacity [75], social isolation [76], anxiety and depressive symptoms, resulting in physical and psychosocial disability [77]. This downward health spiral can go unrecognized and negatively impact HRQL. Interventions should promptly disrupt this downward health spiral and reduce symptom burden, increase physical activity, social engagement, and promote behavior change to improve HRQL and other outcomes. HRQL = health-related quality of life
Our trial supports the vicious cycle of “dyspnea-inactivity” conceptual model and the promise of IMT + walking as a targeted rehabilitative strategy for lung cancer survivors following curative intent therapy. In a small sample, we found that IMT + walking at 1–6 months posttreatment could reduce symptom burden, mitigate the negative impact of symptoms on social functioning and psychological disturbances, and improve HRQL. These potential benefits appeared to be sustained, possibly due to an behavioral support component informed by strong evidence derived from general adult population [35], with supporting evidence among cancer survivors [37, 38], including in a 2023 systematic review [65], and persisted in adjustments for age and comorbidities – two important characteristics in lung cancer. These potential benefits should be confirmed in larger trials with longer follow-up. IMT + walking had no statistically significant benefit on dyspnea, functional exercise capacity, anxiety, or sleep difficulties, possibly due to the small sample size and/or lack of additional targeted strategies (e.g., extremity strength training to increase functional exercise capacity), or in-person supervised exercise sessions. ActivPAL MVPA and SB improved at week 6 but waned at week 12, suggesting that reduced symptom burden could have mediated improved HRQL.
Feasibility was high regarding participant adherence to tele-visits, unsupervised IMT + walking, completion of activity logs and PCOMs, and retention. Enrollment was challenging, although comparable to other exercise trials in cancer [38] and higher than US National Clinical Trials in lung cancer [66], traditionally difficult to enroll [67]. Decreased intervention intensity or additional components, with compelling outcome data aligned with patient values or goals, may be needed for wider uptake. Acceptability to telemedicine-based IMT + walking was also high, with safety complementing a systematic review of in-person exercise training among post-surgical lung cancer survivors [64].
Study strengths, limitations, and future directions
Strengths include: 1) a contemporary sample of lung cancer survivors within 1–6 months following curative intent therapy; 2) evaluation of a novel targeted intervention to disrupt a vicious cycle of “dyspnea-inactivity”; 3) block randomization to ensure balance between groups in a heterogenous population; 4) high feasibility and acceptability; 5) monitoring of safety events that included emergency room visits/hospitalizations in both groups; and 6) telemedicine-based delivery with minimal equipment and interventionist training, enhancing scalability and geographic reach potential.
Study limitations include participant adherence to IMT assessed by self-report, predisposing to social desirability and reporting bias. However, adherence measures completion was high, with step counts obtained by participants from Fitbit devices. Second, the m6MWT has not been evaluated in lung cancer, with responsiveness of the m6MWT distance is not known with certainty, precluding conclusions on feasibility. Nevertheless, functional exercise capacity is associated with HRQL [68], curative intent therapy outcomes, survival [69], is widely-used in exercise/rehabilitation trials [64], and has in-lab MCIDs available in lung cancer [70]. Third, we did not blind the outcome assessor nor use a sham intervention; however, we measured physical activity with the well-validated/highly-accurate activPAL. Fourth, while the vicious cycle of “dyspnea-inactivity” may be an important concept, we did not include other exercise training components, psychosocial interventions, nor strategies to improve sleep/fatigue [71]. Notwithstanding, IMT + walking could be a targeted strategy for dyspneic and physically inactive patients. Fifth, we lack a validated composite HRQL measure for disease-free lung cancer survivors. However, SGRQ subdomains align with the World Health Organization International Classification on Functioning, Disability and Health – a biopsychosocial model that incorporates biological, individual, and social perspectives on health and disability [72]. HRQL measures that include symptoms and treatment effects of advanced/metastatic lung cancer have limited utility for our target population. Sixth, our trial was designed to detect a very-large effect size difference in activPAL steps/day and thus was likely inadequately powered to detect changes in other PCOMs, precluding conclusion on treatment effects. Nevertheless, pilot trials are foundational to efficacy/effectiveness trials [42, 43]. Finally, all participants were from one healthcare system, most of white race/ethnicity, with lower-than-expected COPD prevalence, limiting generalizability.
Future trials can consider baseline dyspnea and physical activity levels to reduce participant heterogeneity, investigation of biophysiological mechanisms relating dyspnea and IMT, incorporate additional components, possibly with novel randomized trial designs (e.g., multiphase optimization strategy, hybrid, adaptive, platform) to encourage uptake, completion, and clinical translation.
Conclusion
We conclude that telemedicine-based IMT + walking is feasible, acceptable, safe, and could disrupt the vicious cycle of “dyspnea-inactivity” downward health spiral among lung cancer survivors following curative intent therapy. These results warrant further investigations, including in prospective observational studies with longer follow-up, and larger, adequately powered randomized clinical trials.
Abbreviations
- BDS:
-
Borg Dyspnea Score
- BMI:
-
Body-mass index
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- ED:
-
Emergency department
- FEV1 :
-
Forced expiratory volume in 1 s
- FVC:
-
Forced vital capacity
- GAD-7:
-
Generalized Anxiety Disorder 7-item
- HRQL:
-
Health-related quality of life
- IMT:
-
Inspiratory muscle training
- IPAQ-SF:
-
International Physical Activity Questionnaire – Short Form
- KPCO:
-
Kaiser Permanente Colorado
- LPA:
-
Light intensity physical activity
- m6MWT:
-
Mobile-based six-minute walk test
- MCID:
-
Minimal clinically important difference
- MVPA:
-
Moderate-vigorous intensity physical activity
- NSCLC-NOS:
-
Non-small cell lung cancer, not otherwise specified
- PA:
-
Physical activity
- PAGAC:
-
Physical Activity Guidelines Advisory Committee
- PCOM:
-
Patient-centered outcome measure
- PSQI:
-
Pittsburgh Sleep Quality Index
- RS:
-
Research specialist
- SB:
-
Sedentary behavior
- SBRT:
-
Stereotactic body radiotherapy
- SD:
-
Standard deviation
- SCT:
-
Social Cognitive Theory
- SES:
-
Standardized effect size
- SGRQ:
-
St. George’s Respiratory Questionnaire
- SOBQ:
-
University of California Shortness of Breath Questionnaire
References
Kathiresan G, Clement RF, Sankaranarayanan MT (2010) Dyspnea in lung cancer patients: a systematic review. Lung Cancer (Auckl) 1:141–150
Ha D, Ries AL (2020) Characterization of Dyspnea in Veteran Lung Cancer Survivors Following Curative-Intent Therapy. J Cardiopulm Rehabil Prev 40(2):120–127
Ban WH, Lee JM, Ha JH et al (2016) Dyspnea as a Prognostic Factor in Patients with Non-Small Cell Lung Cancer. Yonsei Med J 57(5):1063–1069
Ha D, Ries AL, Lippman SM et al (2020) Effects of curative-intent lung cancer therapy on functional exercise capacity and patient-reported outcomes. Support Care Cancer 28(10):4707–4720
Wang Q, Stone K, Kern JA et al (2022) Adverse Events Following Limited Resection versus Stereotactic Body Radiation Therapy for Early-Stage Lung Cancer. Ann Am Thorac Soc 19(12):2053–2061
Cella L, Monti S, Thor M et al (2021) Radiation-Induced Dyspnea in Lung Cancer Patients Treated with Stereotactic Body Radiation Therapy. Cancers (Basel) 13(15):3743
Li JJ, Li JR, Wu JM et al (2021) Change in symptom clusters perioperatively in patients with lung cancer. Eur J Oncol Nurs 55:102046
Poghosyan H, Sheldon LK, Leveille SG et al (2013) Health-related quality of life after surgical treatment in patients with non-small cell lung cancer: a systematic review. Lung Cancer (Amsterdam, Netherlands) 81(1):11–26
Ubels RJ, Mokhles S, Andrinopoulou ER et al (2015) Quality of life during 5 years after stereotactic radiotherapy in stage I non-small cell lung cancer. Radiat Oncol 10:98
Gralla RJ, Hollen PJ, Msaouel P et al (2014) An evidence-based determination of issues affecting quality of life and patient-reported outcomes in lung cancer: results of a survey of 660 patients. J Thorac Oncol 9(9):1243–1248
Undem BJ, Kollarik M (2005) The role of vagal afferent nerves in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2(4):355–60 (discussion 71-2)
Adriaensen D, Timmermans JP (2011) Breath-taking complexity of vagal C-fibre nociceptors: implications for inflammatory pulmonary disease, dyspnoea and cough. J Physiol 589(Pt 1):3–4
O’Donnell DE, Banzett RB, Carrieri-Kohlman V et al (2007) Pathophysiology of dyspnea in chronic obstructive pulmonary disease: a roundtable. Proc Am Thorac Soc 4(2):145–168
Koczywas M, Williams AC, Cristea M et al (2013) Longitudinal changes in function, symptom burden, and quality of life in patients with early-stage lung cancer. Ann Surg Oncol 20(6):1788–1797
Ostroff JS, Krebs P, Coups EJ et al (2011) Health-related quality of life among early-stage, non-small cell, lung cancer survivors. Lung Cancer (Amsterdam, Netherlands) 71(1):103–108
Ha DM, Prochazka AV, Bekelman DB et al (2022) Modifiable factors associated with health-related quality of life among lung cancer survivors following curative intent therapy. Lung Cancer 163:42–50
Parshall MB, Schwartzstein RM, Adams L et al (2012) An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 185(4):435–452
Granger CL, Connolly B, Denehy L et al (2017) Understanding factors influencing physical activity and exercise in lung cancer: a systematic review. Support Care Cancer 25(3):983–999
Teba PP, Esther MG, Raquel SG (2022) Association between physical activity and patient-reported outcome measures in patients with lung cancer: a systematic review and meta-analysis. Qual Life Res 31(7):1963–1976
Yang JJ, Yu D, White E et al (2022) Prediagnosis Leisure-Time Physical Activity and Lung Cancer Survival: A Pooled Analysis of 11 Cohorts. JNCI Cancer Spectr 6(2):pkac009.
Colt HG, Murgu SD, Korst RJ et al (2013) Follow-up and surveillance of the patient with lung cancer after curative-intent therapy: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143(5 Suppl):e437S – e454
Siegel RL, Miller KD, Fuchs HE et al (2021) Cancer Statistics, 2021. CA: Cancer J Clin 71(1):7–33
Ammous O, Feki W, Lotfi T et al (2023) Inspiratory muscle training, with or without concomitant pulmonary rehabilitation, for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev 1(1):Cd013778
Driessen EJ, Peeters ME, Bongers BC et al (2017) Effects of prehabilitation and rehabilitation including a home-based component on physical fitness, adherence, treatment tolerance, and recovery in patients with non-small cell lung cancer: A systematic review. Crit Rev Oncol Hematol 114:63–76
Wong JN, McAuley E, Trinh L (2018) Physical activity programming and counseling preferences among cancer survivors: a systematic review. Int J Behav Nutr Phys Act 15:1–21
Czajkowski SM, Powell LH, Adler N et al (2015) From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychol 34(10):971–982
Harris PA, Taylor R, Thielke R et al (2009) Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381
Eldridge SM, Chan CL, Campbell MJ et al (2016) CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 355:i5239
Spruit MA, Singh SJ, Garvey C et al (2013) An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 188(8):e13-64
Beaumont M, Forget P, Couturaud F et al (2018) Effects of inspiratory muscle training in COPD patients: A systematic review and meta-analysis. Clin Respir J 12(7):2178–2188
Tudor-Locke CE, Myers AM, Bell RC et al (2002) Preliminary outcome evaluation of the First Step Program: a daily physical activity intervention for individuals with type 2 diabetes. Patient Educ Couns 47(1):23–28
Schmitz KH, Campbell AM, Stuiver MM et al (2019) Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA: Cancer J Clin 69(6):468–484
Breathlessness - Shortness of Breath (2020) American thoracic society patient education/information series [Available from: https://www.thoracic.org/patients/patient-resources/resources/breathlessness.pdf. Accessed Dec 2021
Physical Activity and Lung Cancer (2021). American lung association [Available from: https://www.lung.org/lung-health-diseases/lung-disease-lookup/lung-cancer/treatment/stay-healthy/physical-activity-and-lung-cancer. Accessed Dec 2021
Physical Activity Guidelines Advisory Committee Scientific Report (2018) Physical activity guidelines advisory committee. Washington, DC: U.S. Department of Health and Human Services. https://health.gov/paguidelines/second-edition/report/pdf/PAG_Advisory_Committee_Report.pdf
Bandura A (2004) Health promotion by social cognitive means. Health Educ Behav 31(2):143–164
Stacey FG, James EL, Chapman K et al (2015) A systematic review and meta-analysis of social cognitive theory-based physical activity and/or nutrition behavior change interventions for cancer survivors. J Cancer Survivorship : Res Pract 9(2):305–338
Turner RR, Steed L, Quirk H et al (2018) Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev 9:CD010192
O’Halloran PD, Blackstock F, Shields N et al (2014) Motivational interviewing to increase physical activity in people with chronic health conditions: a systematic review and meta-analysis. Clin Rehabil 28(12):1159–1171
Sit Less Move More (2020) American college of sports medicine [Available from: https://www.exerciseismedicine.org/assets/page_documents/EIM_Rx%20for%20Health_Sit%20Less%20Move%20More.pdf. Accessed Dec 2021
Being Active for a Better Life (2020) American college of sports medicine [Available from: https://www.exerciseismedicine.org/assets/page_documents/EIM_Rx%20for%20Health_Being%20Active%20for%20a%20Better%20Life.pdf. Accessed Dec 2021
Eldridge SM, Lancaster GA, Campbell MJ et al (2016) Defining Feasibility and Pilot Studies in Preparation for Randomised Controlled Trials: Development of a Conceptual Framework. PLoS ONE 11(3):e0150205
El-Kotob R, Giangregorio LM (2018) Pilot and feasibility studies in exercise, physical activity, or rehabilitation research. Pilot Feasibility Stud 4(1):1–7
Holland AE, Cox NS, Houchen-Wolloff L et al (2021) Defining Modern Pulmonary Rehabilitation. An Official American Thoracic Society Workshop Report. Ann Am Thorac Soc 18(5):12–29
Buffart LM, Kalter J, Sweegers MG et al (2017) Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev 52:91–104
Bakken S, Grullon-Figueroa L, Izquierdo R et al (2006) Development, validation, and use of English and Spanish versions of the telemedicine satisfaction and usefulness questionnaire. J Am Med Informa Assoc: JAMIA 13(6):660–667
Edwardson CL, Winkler EAH, Bodicoat DH et al (2017) Considerations when using the activPAL monitor in field-based research with adult populations. J Sport Health Sci 6(2):162–178
Lynch BM, Nguyen NH, Moore MM et al (2019) A randomized controlled trial of a wearable technology-based intervention for increasing moderate to vigorous physical activity and reducing sedentary behavior in breast cancer survivors: The ACTIVATE Trial. Cancer 125(16):2846–2855
Gomersall SR, Skinner TL, Winkler E et al (2019) Feasibility, acceptability and efficacy of a text message-enhanced clinical exercise rehabilitation intervention for increasing “whole-of-day” activity in people living with and beyond cancer. BMC Public Health. 19(Suppl 2):1–4
Maddocks M, Wilcock A (2012) Exploring physical activity level in patients with thoracic cancer: implications for use as an outcome measure. Support Care Cancer 20(5):1113–1116
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories (2002) ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 166(1):111–7
Salvi D, Poffley E, Orchard E et al (2020) The Mobile-Based 6-Minute Walk Test: Usability Study and Algorithm Development and Validation. JMIR Mhealth Uhealth 8(1):e13756
Mak J, Rens N, Savage D et al (2021) Reliability and repeatability of a smartphone-based 6-min walk test as a patient-centred outcome measure. Eur Heart J Digit Health 2(1):77–87
Salvi D, Poffley E, Tarassenko L et al (2021) App-Based Versus Standard Six-Minute Walk Test in Pulmonary Hypertension: Mixed Methods Study. JMIR Mhealth Uhealth 9(6):e22748
Enright PL, Sherrill DL (1998) Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 158(5 Pt 1):1384–1387
Whitehead AL, Julious SA, Cooper CL et al (2016) Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res 25(3):1057–1073
Teylan M, Kantorowski A, Homsy D et al (2019) Physical activity in COPD: Minimal clinically important difference for medical events. Chron Respir Dis 16:1479973118816424
Quan H, Sundararajan V, Halfon P et al (2005) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43(11):1130–1139
From Cancer Patient to Cancer Survivor: Lost in transition. Washington, DC: the national academies press: institute of medicine and national research council, 2006. (Report) https://nap.nationalacademies.org/catalog/11468/from-cancer-patient-to-cancer-survivor-lost-in-transition
Patel AV, Friedenreich CM, Moore SC et al (2019) American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med Sci Sports Exerc 51(11):2391–2402
Rock CL, Thomson CA, Sullivan KR et al (2022) American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin 72(3):230–262
Campbell KL, Winters-Stone KM, Wiskemann J et al (2019) Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc 51(11):2375–2390
Avancini A, Sartori G, Gkountakos A et al (2020) Physical Activity and Exercise in Lung Cancer Care: Will Promises Be Fulfilled? Oncologist 25(3):e555–e569
Cavalheri V, Burtin C, Formico VR et al (2019) Exercise training undertaken by people within 12 months of lung resection for non-small cell lung cancer. Cochrane Database Syst Rev 6:CD009955
Rodrigues B, Carraça EV, Francisco BB et al (2023) Theory-based physical activity and/or nutrition behavior change interventions for cancer survivors: a systematic review. J Cancer Surviv (In eng). https://doi.org/10.1007/s11764-023-01390-5
Pang HH, Wang X, Stinchcombe TE et al (2016) Enrollment Trends and Disparity Among Patients With Lung Cancer in National Clinical Trials, 1990 to 2012. J Clin Oncol 34(33):3992–3999
Spiro SG, Gower NH, Evans MT et al (2000) Recruitment of patients with lung cancer into a randomised clinical trial: experience at two centres. On behalf of the Big Lung Trial Steering Committee. Thorax. 55(6):463–5
Ha D, Ries AL, Mazzone PJ et al (2018) Exercise capacity and cancer-specific quality of life following curative intent treatment of stage I-IIIA lung cancer. Support Care Cancer 26(7):2459–2469
Ha D, Mazzone PJ, Ries AL et al (2016) The Utility of Exercise Testing in Patients with Lung Cancer. J Thorac Oncol 11(9):1397–1410
Granger CL, Holland AE, Gordon IR et al (2015) Minimal important difference of the 6-minute walk distance in lung cancer. Chron Respir Dis 12(2):146–154
Bade BC, Faiz SA, Ha DM et al (2023) Cancer-related Fatigue in Lung Cancer: A Research Agenda: An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med 207(5):e6–e28
ICF Beginner's Guide (2002) Towards a common language for functioning, disability and health world health organization [Available from: https://www.who.int/publications/m/item/icf-beginner-s-guide-towards-a-common-language-for-functioning-disability-and-health. Accessed Dec 2021
Zheng W, Hu M, Liu Y (2022) Social support can alleviate the fear of cancer recurrence in postoperative patients with lung carcinoma. Am J Transl Res 14(7):4804–4811
Granger CL, Parry SM, Edbrooke L et al (2016) Deterioration in physical activity and function differs according to treatment type in non-small cell lung cancer - future directions for physiotherapy management. Physiotherapy 102(3):256–263
Granger CL, McDonald CF, Irving L et al (2014) Low physical activity levels and functional decline in individuals with lung cancer. Lung Cancer (Amsterdam, Netherlands) 83(2):292–299
Zhang N, He X, Zhang H et al (2022) Influencing Factors of Physical Activity in Patients with Lung Cancer Surgery and Its Correlation with Exercise Self-Efficacy and Perceived Social Support. Evid Based Complement Alternat Med 2022:7572530
Joshy G, Thandrayen J, Koczwara B et al (2020) Disability, psychological distress and quality of life in relation to cancer diagnosis and cancer type: population-based Australian study of 22,505 cancer survivors and 244,000 people without cancer. BMC Med 18(1):1–15
Funding
This work was supported by the American Lung Association, Lung Cancer Discovery Award (820773, PI Ha). The American Lung Association had no role in the study design, data collection, analysis, or interpretation, writing, or decision to submit the article for publication. Dr. Ha is also supported by the Career Development Award (1IK2RX003661) from the United States (U.S.) Department of Veterans Affairs, Rehabilitation Research and Development Service. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs, the U.S. government, or the American Lung Association.
Author information
Authors and Affiliations
Contributions
DMH, AC, BD, RB, MF, CZ, and RSB contributed to the study conception, design, conduct, data collection, analysis, interpretation, and/or manuscript preparation. WSG, JJA, HJL, AM, AVP, and RLK contributed to the interpretation and critical appraisal of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki and approved by the Kaiser Permanente Institutional Review Board (1717517-12).
Consent to participate
Informed consent was obtained from all individual participants.
Consent to publish
The authors affirm that participants provided informed consent for publication. No patient identifying information is included in this manuscript.
Conflict of interest
All authors declare that no conflict of interest exists. Dr. Malhotra is funded by NIH. He reports income related to medical education from Jazz, Zoll, Eli Lilly and Livanova. ResMed provided a philanthropic donation to the University of California San Diego.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ha, D.M., Comer, A., Dollar, B. et al. Telemedicine-based inspiratory muscle training and walking promotion with lung cancer survivors following curative intent therapy: a parallel-group pilot randomized trial. Support Care Cancer 31, 546 (2023). https://doi.org/10.1007/s00520-023-07999-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-07999-7